Pharmacovigilance Outsourcing Market size worth $10.5 Bn by 2026
Published Date: March 2020
Pharmacovigilance (PV) Outsourcing Market size will surpass USD 10.5 billion by 2026, according to a new research report by Global Market Insights Inc.
Pharmacovigilance (PV) outsourcing market is primarily driven by the benefits offered by outsourced services providers over in-house pharmacovigilance department. The global pharmaceutical industry is witnessing robust growth owing to advancements in drug discovery process leading to new product launch. Regulatory agencies play a vital role in managing and ensuring safety parameters of these products. It is thus, mandatory for the pharma and biotech companies to focus on PV activities being performed in-house or outsourced. Setting up in-house PV department involves a lot of costs and challenges thus, increasing the demand for CROs and BPOs to outsource PV activities.
High profile drug recalls owing to safety concerns will foster the PV outsourcing market growth
Increasing investments in R&D activities has led to development of numerous novel products. Advancements in drug discovery process has further resulted in increased number of products being launched across the global market. This growth potential leads to a substantial increase in the number of adverse drug reactions and other safety concerns associated with these products. Focus of pharmaceutical companies on identifying and minimizing the risk of hazardous drug interactions will further accelerate the pharmacovigilance outsourcing market revenue. However, risks associated with data security in clinical studies may restrain the pharmacovigilance outsourcing industry growth through 2026.
Get more details on this report - Request Free Sample PDF
Medical burden of adverse drug reactions will upsurge the demand for post marketing services
The pharmacovigilance outsourcing services market is segmented into pre-marketing, post-marketing and other services. Safety data management services, clinical pharmacovigilance services, medical review and case processing services are included in the pre-marketing services segment. The post-marketing services include knowledge process outsourcing services as well as IT solutions and services.
The pharmacovigilance outsourcing market from post-marketing services segment accounted for more than USD 2.7 billion in 2019 and is projected to witness lucrative CAGR over the analysis period. Segment growth is attributed to increasing demand for PV services to assess the safety of products after its release in the market. Adoption of post-marketing pharmacovigilance services to identify actual clinical use and interactions of newly developed drugs in the diverse population will thus, spur the market size.
Browse key industry insights spread across 225 pages with 199 market data tables & 8 figures & charts from the report, “Pharmacovigilance Outsourcing Market Size By Service (Pre-marketing Services {Clinical Services, Case-Processing Services, Safety Data Management Services, Medical Review}, Post-marketing Services {Pharmacovigilance Knowledge Process Outsourcing Services, IT Solutions and Services}), By Service Providers (Contract Research Organizations, Business Processing Outsourcing), Regional Outlook, Price Trends, Application Potential, Competitive Market Share & Forecast, 2020 – 2026” in detail along with the table of contents: https://www.gminsights.com/industry-analysis/pharmacovigilance-outsourcing-market
Usage of advanced software to deliver superior quality PV report will pave way for business process outsourcing organizations growth
The BPOs segment held significant pharmacovigilance outsourcing market share in 2019 and is estimated to show over 15% CAGR during 2020 to 2026. Business process outsourcing organizations outsource knowledge-based pharmacovigilance processes such as case processing, benefit risk management, medical review as well as safety surveillance. Focus of BPOs on incorporating advanced technology within the software for ensuring complete patient safety, minimizing adverse drug events and generating superior quality PV reports will prove beneficial for the market growth.
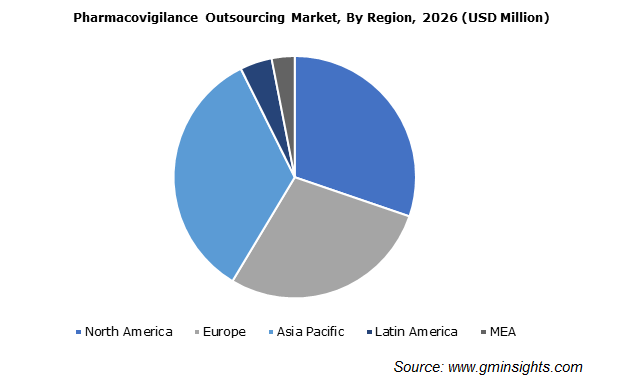
Asia Pacific is the hotbed for outsourcing pharmacovigilance services
Asia Pacific pharmacovigilance outsourcing market demand is anticipated to surpass USD 3.6 billion by 2026. Regional industry growth is attributed to increasing pressure on pharmaceutical companies regarding proactive detection and management of safety signals.
Intense safety regulations laid by the regulatory bodies across the world, in a bid to endure consumer safety will increase the demand for PV activities being outsourced in Asian countries such as India and China. Moreover, availability of skilled healthcare professionals in these countries, offering cost-effective PV services will thus, foster the regional market progress.
Growing preference towards outsourcing PV activities will offer growth opportunities to the business players
Some of the eminent players operating in pharmacovigilance outsourcing market share include BioClinica, Accenture, Covance, ICON, Cognizant, iGATE Corporation, Infosys, Genpact, TCS, PAREXEL, Syneos Health and Oracle. These industry players focus on various organic and inorganic strategic such as partnerships, acquisitions, mergers and development of innovative PV platforms to garner more revenue and sustain industry competition. For instance, in May 2017, Medpace renewed its Global Impact Partnership with the Society for Clinical Research Sites (SCRS), that enabled Medpace to better access the investigative sites for successful clinical trials and capitalize on market opportunities.





