Home > Healthcare > Medical Devices > Surgical Devices > Thrombectomy Devices Market
Thrombectomy Devices Market Analysis
- Report ID: GMI5245
- Published Date: Sep 2023
- Report Format: PDF
Thrombectomy Devices Market Analysis
Thrombectomy devices market from mechanical/fragmentation segment size is estimated to record USD 840 million by 2032 owing to expanding usage to offer improved efficacy in the treatment of diseases. Moreover, the rise in R&D investments by industry players for introducing highly advanced mechanical thrombectomy devices integrated with advanced computer-aided components will add to the segment progress. To cite an instance, in January 2023, Penumbra, Inc. announced the approval and launch of Lightning FlashTM, the most advanced and powerful mechanical thrombectomy system featuring an advanced microprocessor algorithm.
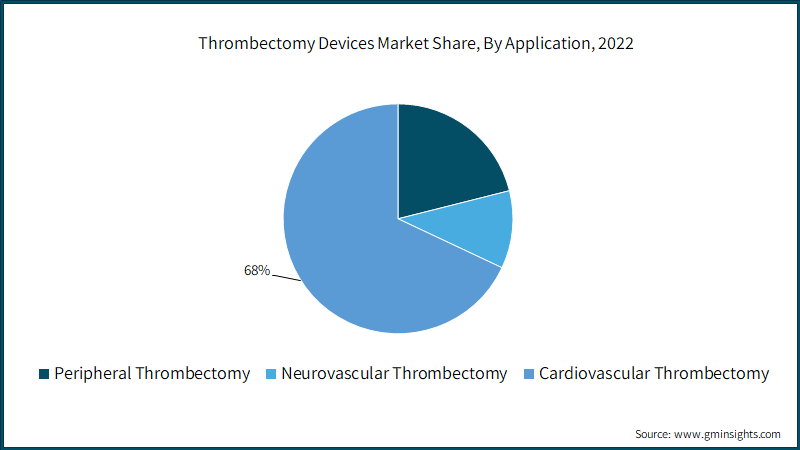
Thrombectomy devices market from the cardiovascular thrombectomy application segment is likely to witness around 7.3% CAGR from 2023 to 2032. The growth is attributed to the surge in the number of patients suffering from severe heart conditions. According to research published in the Journal of the American College of Cardiology in August 2022, the estimated rate of cardiovascular risk factors and disease in the U.S. would rise considerably by 2060. Significant increases in cardiovascular trends may add to the burden on the American healthcare system and highlight the need for fair access to early disease preventive education and treatment options, subsequently surging the need for cardiovascular thrombectomy.
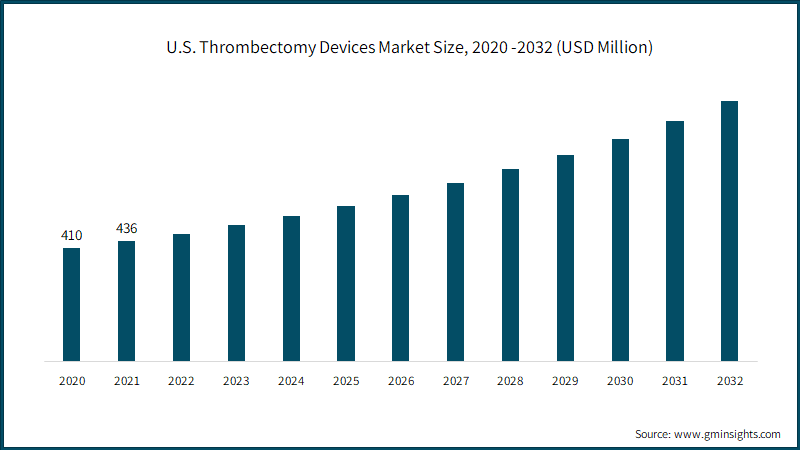
North America thrombectomy devices market size is expected to expand at 7.4% CAGR from 2023-2032 driven by the growing awareness regarding vascular diseases, increasing healthcare expenditure, favorable reimbursement scenarios, and government initiatives. The rapidly expanding geriatric population base highly prone to several chronic disorders along with the increasing preference for non-surgical treatments has pushed several manufacturers to introduce medically advanced devices, leading to enhanced regional industry growth. For instance, in June 2023, Surmodics, Inc., announced that it had received the U.S. FDA 510(k) clearance for its Pounce™ LP Thrombectomy System designed for the non-surgical removal of thrombi and emboli from the peripheral arterial vasculature.

