Home > Healthcare > Medical Devices > Diagnostic Devices > Respiratory Disease Testing Market
Respiratory Disease Testing Market Analysis
- Report ID: GMI1871
- Published Date: Apr 2023
- Report Format: PDF
Respiratory Disease Testing Market Analysis
Based on test type, the respiratory disease testing market size from in vitro diagnostics tests is anticipated to showcase more than 6% CAGR from 2023-2032. In vitro diagnostics (IVD) respiratory tests or laboratory-based tests are extensively adopted as they render multiple advantages for the diagnosis and treatment of respiratory conditions. Some of these include delivering both non-invasive as well as customizable accurate and rapid results, while ensuring that healthcare providers are offering effective, affordable and personalized care. IVD respiratory tests also help to analyse the samples of bodily fluids, including blood, sputum, or urine for detecting and diagnosing the respiratory conditions.

Respiratory disease testing market share from the hospitals end-use segment will exceed USD 10 billion by 2032 due to the strong availability of advanced medical equipment and treatment options. Hospitals are increasingly favoured by patients as they offer better interaction of healthcare professionals on account of ease of accessibility. The growing influx of technologically advanced devices to treat the rising respiratory ailments across the hospital settings in the developed as well as developing countries will anchor the market development.
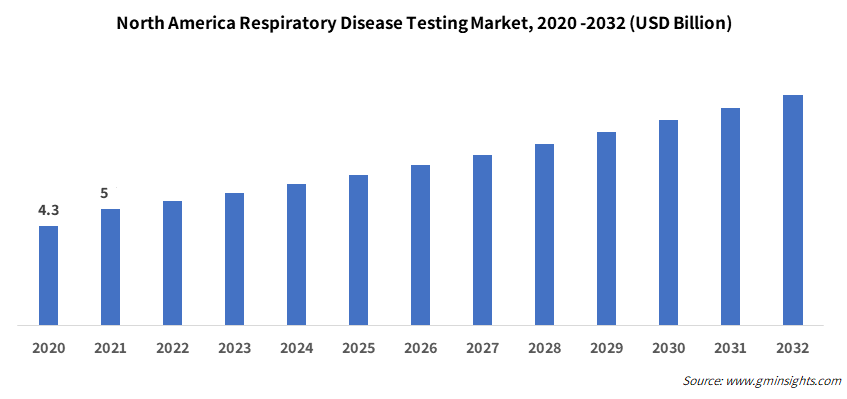
North America respiratory disease testing market size is anticipated to strike 6.4% CAGR from 2023-2032. The growth can be attributed to the presence of well-developed healthcare infrastructure in the region. The robust presence of a large number of business players has spurred the introduction of diverse product offerings. The rising burden of respiratory diseases will further anchor the product adoption. As per CDC (Centers for Disease Control and Prevention), nearly 4.6% of adults were diagnosed with emphysema, COPD, or chronic bronchitis in the U.S. in 2021.

