Summary
Table of Content

Rare Disease Treatment Market
Get a free sample of this report
Form submitted successfully!
Error submitting form. Please try again.
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!

Request Sectional Data
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!
Form submitted successfully!
Error submitting form. Please try again.
Rare Disease Treatment Market Size
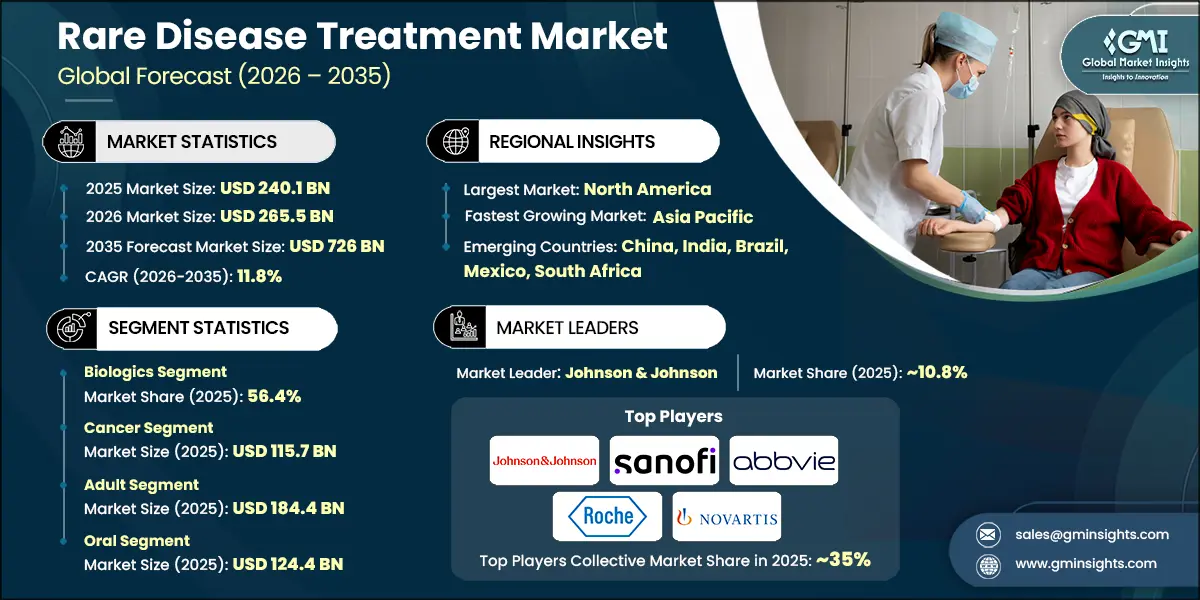
The global rare disease treatment market size was valued at USD 240.1 billion in 2025. The market is expected to grow from USD 265.5 billion in 2026 to USD 726 billion in 2035, growing at a CAGR of 11.8% during the forecast period, according to the latest report published by Global Market Insights Inc.
To get key market trends
The market is primarily driven by the high prevalence of rare genetic and metabolic disorders. For instance, the Lancet mentions that more than 300 million people live with rare diseases globally, escalating demand for treatment options. Moreover, Rare Disease International states that there are approximately 6,000 clinically recognized rare diseases globally, with 72% being genetic, and 70% of cases occurring in childhood.
Additionally, advancements in precision medicine and supportive regulatory frameworks, such as orphan drug designations, further stimulate the market growth. For instance, more than 50% of the new drug approvals are found to be orphan drugs, which have surged significantly, reflecting the growing focus on rare disease therapies. The rising adoption of gene therapies, enzyme replacement therapy, and targeted biologics further stimulates market expansion.
Rare Disease Treatment Market Report Attributes
| Key Takeaway | Details |
|---|---|
| Market Size & Growth | |
| Base Year | 2025 |
| Market Size in 2025 | USD 240.1 Billion |
| Market Size in 2026 | USD 265.5 Billion |
| Forecast Period 2026 - 2035 CAGR | 11.8% |
| Market Size in 2035 | USD 726 Billion |
| Key Market Trends | |
| Drivers | Impact |
| Increasing prevalence of rare diseases | Rising cases of genetic disorders, metabolic syndromes, and autoimmune conditions are increasing demand for specialized therapies. |
| Advancements in precision medicine and biotechnology | Breakthroughs in gene therapy and targeted biologics are improving treatment outcomes and expanding therapeutic options for rare disease patients. |
| Supportive regulatory frameworks and incentives | Orphan drug designations, fast-track approvals, and tax benefits provided by regulatory agencies are increasing the commercialization of rare disease treatments. |
| Growing awareness and early diagnosis rates | Improved screening programs, genetic testing, and patient advocacy initiatives are increasing diagnosis rates and access to advanced therapies worldwide. |
| Pitfalls & Challenges | Impact |
| High cost and limited affordability | Rare disease treatments, especially gene and cell therapies, involve complex development and manufacturing processes, making them prohibitively expensive for many patients. |
| Small patient populations and clinical trial complexity | Recruiting patients for trials is challenging due to low prevalence, leading to longer development cycles and higher costs. |
| Opportunities: | Impact |
| Expansion into emerging markets with improving healthcare infrastructure | Rising healthcare investments in Asia-Pacific, Latin America, and the Middle East will create significant growth potential for rare disease treatments. |
| Collaborations and digital health integration | Partnerships between pharma companies, biotech firms, and digital health platforms will enhance patient engagement, remote monitoring, and therapy adherence. |
| Market Leaders (2025) | |
| Market Leaders |
~10.8% market share |
| Top Players |
|
| Competitive Edge |
|
| Regional Insights | |
| Largest Market | North America |
| Fastest growing market | Asia Pacific |
| Emerging countries | China, India, Brazil, Mexico, South Africa |
| Future outlook |
|
What are the growth opportunities in this market?
Rare disease treatments encompass a wide range of therapeutic approaches aimed at managing or curing conditions that affect a small patient population. These treatments include orphan drugs, gene therapies, biologics, and small molecules, which often require specialized development and regulatory pathways. Key players driving market growth include Sanofi, AstraZeneca, F. Hoffmann-La Roche, Vertex Pharmaceuticals, Pfizer, and AbbVie, which are actively investing in research and development, strategic collaborations, and global distribution networks to address unmet needs in rare disease care. These companies play a critical role in innovation, patient access programs, and expanding treatment availability worldwide.
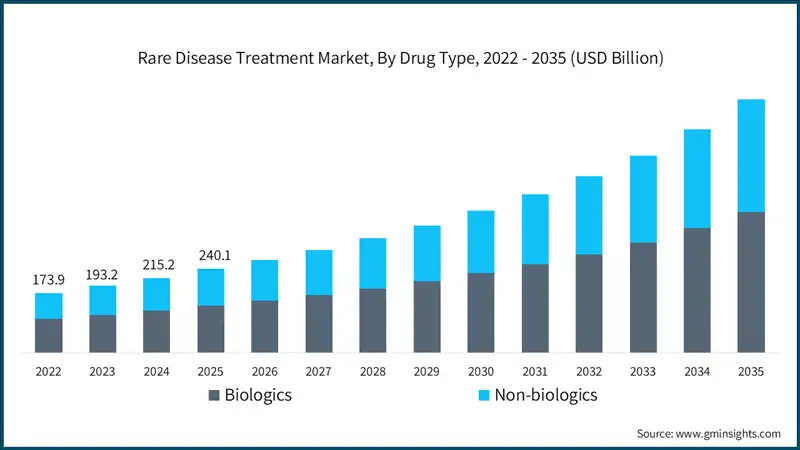
Between 2022 and 2024, the rare disease treatment market experienced notable growth, driven by increased regulatory incentives, rising awareness, and breakthroughs in advanced therapies. For instance, the global market was valued at about USD 173.9 billion in 2022 and reached around USD 215.2 billion in 2024, growing at a moderate CAGR of approximately 11.2%. This growth can be attributed to the increasing number of orphan drug approvals, rapid adoption of gene and cell therapies, and the emergence of precision medicine approaches that improve treatment outcomes for rare disease patients.
The market refers to the global industry focused on therapies for conditions with low prevalence but high unmet medical needs. These treatments aim to improve survival, quality of life, and disease management through innovative solutions such as orphan drugs, advanced biologics, and genetic interventions, often supported by favorable regulatory and reimbursement frameworks.
Rare Disease Treatment Market Trends
- A major trend in the market is the growing shift toward advanced genetic and molecular therapies. Traditional symptomatic treatments are increasingly being replaced by gene therapies, RNA-based drugs, and targeted biologics that address the root cause of rare conditions. These approaches offer long-term efficacy, potential cures, and reduced dependency on chronic medication, making them highly attractive for both patients and healthcare providers.
- Additionally, there is a rising adoption of personalized and precision medicine solutions. With advancements in genomic sequencing and biomarker identification, treatments are being tailored to individual patient profiles, improving therapeutic outcomes and minimizing adverse effects. This trend aligns with the broader healthcare movement toward patient-centric care, particularly for rare diseases where variability in genetic expression significantly impacts treatment success.
- Furthermore, the increasing prevalence of rare genetic disorders and improved diagnostic capabilities are major catalysts for market growth. As global life expectancy rises and genetic testing becomes more accessible, the number of diagnosed rare disease cases continues to expand across both developed and emerging regions.
- For instance, according to the World Health Organization (WHO), rare diseases collectively affect over 300 million people worldwide, with approximately 7,000 identified conditions, many of which lack approved treatments. This growing patient pool underscores the urgent need for innovative therapies.
- In addition, regulatory support and orphan drug incentives are accelerating innovation and commercialization. Regulatory bodies such as the FDA and EMA have granted thousands of orphan drug designations over the past decade, offering benefits like market exclusivity, tax credits, and expedited approvals. For example, as per the National Institute of Health (NIH), as of December 31, 2022, the U.S. FDA’s Office of Orphan Products Development had granted more than 6,340 orphan drug designations since the program began in 1983, covering over 1,000 rare diseases.
- Moreover, technological innovations such as CRISPR-based gene editing and RNA interference therapies are gaining traction. These cutting-edge modalities enable precise genetic modifications and targeted silencing of disease-causing genes, paving the way for curative treatments. Such breakthroughs are particularly important for conditions like spinal muscular atrophy, hemophilia, and lysosomal storage disorders, where conventional therapies offer limited efficacy.
- Thus, as global research and development investments surge and healthcare infrastructure improves, the market is poised for sustained expansion. The growing clinical emphasis on curative approaches, early intervention, and improved quality of life for patients with high unmet needs will continue to drive innovation and adoption worldwide.
Rare Disease Treatment Market Analysis
Learn more about the key segments shaping this market
Based on the drug type, the global market is segmented into biologics and non-biologics. The biologics segment has asserted its dominance in the market by securing a significant market share of 56.4% in 2025 and is anticipated to grow at a CAGR of 11.7% over the forecast years.
- Biologics are known to be disease-modifying solutions for more complex genetic and immunological disorders, making them the preferred choice for rare conditions.
- For instance, as per the Applied Clinical Trials analysis (2025) estimates, 35% of all drugs and biologics in the global R&D pipeline target rare diseases, illustrating a broad and growing commitment, especially in biologic and advanced modality development.
- Moreover, biologics have become the preferred and primary choice for rare disease treatment as they provide precision, along with reduced adverse effects, and potential for curative outcomes.
- For instance, gene therapies, including onasemnogene abeparvovec for diseases such as spinal muscular atrophy and monoclonal antibodies for rare immunological disorders, have transformed treatment paradigms.
- Additionally, biologics are increasingly being developed in personalized formats, leveraging genomic data and biomarkers to tailor therapies to individual patients. This trend aligns with the growing emphasis on precision medicine and patient-centric care in rare disease management.
- On the other hand, non-biologics, including small molecules and traditional drugs, hold a smaller share of the market but remain relevant for certain rare conditions where biologics are not yet available or feasible.
Based on the therapeutic area, the global rare disease treatment market is classified into cancer, blood-related disorders, central nervous system, respiratory disorders, musculoskeletal disorders, cardiovascular disorders, and other therapeutic areas. The cancer segment accounted for the highest market revenue of USD 115.7 billion in 2025.
- The high prevalence of rare cancers such as neuroblastoma, desmoid tumors, and certain hematologic malignancies represents a significant portion of rare disease cases globally.
- For example, as per the International Agency for Research on Cancer, rare cancers account for about 25-30% of all cancer diagnoses and 25% of cancer deaths, representing a substantial burden of disease. About 5.1 million people in the European Union and UK are affected by rare cancers, and more than 650 000 new cases are diagnosed per year. These conditions often lack effective standard treatments, creating strong demand for innovative therapies and orphan drugs.
- Moreover, the cancer segment benefits from a strong pipeline of targeted biologics, immunotherapies, and gene-based treatments. Breakthrough modalities such as CAR-T cell therapies, monoclonal antibodies, and precision oncology drugs are transforming treatment paradigms for rare tumors, offering improved survival rates and better quality of life.
- On the other hand, blood-related disorders represent the fastest-growing segment in the market, and are expected to grow at a CAGR of 12.3% during the analysis period.
- Rare conditions such as hemophilia, sickle cell disease, and immunodeficiency disorders are witnessing increased diagnosis rates and treatment adoption, thereby driving demand for innovative treatment options.
Based on the patient, the global rare disease treatment market is classified into adult and pediatric. The adult segment accounted for the highest market revenue of USD 184.4 billion in 2025 and is projected to grow at a CAGR of 11.7% during the analysis period. On the other hand, the pediatric segment, while smaller, is witnessing high growth, escalated by expanded newborn screening programs, early genetic testing, and rising awareness among healthcare providers and caregivers.
- Adults dominated the market in 2025, primarily due to the higher prevalence of rare cancers, rare cardiovascular disorders, and autoimmune conditions among the adult population.
- Moreover, many rare diseases either manifest later in life or progress from childhood into adulthood, making adults the largest treatment group globally. Also, increased diagnosis rates in adults, coupled with greater access to advanced therapies such as biologics, gene therapies, and precision medicine approaches, have significantly contributed to this segment’s leadership.
Based on the route of administration, the global rare disease treatment market is classified into oral, injectable and other routes of administration. The oral segment accounted for the highest market revenue of USD 124.4 billion in 2025.
- Oral drugs hold the largest share due to their convenience, ease of administration, and strong patient compliance, especially for chronic rare conditions requiring long-term therapy.
- Many small-molecule orphan drugs and targeted therapies for rare cancers, metabolic disorders, and immunological conditions are available in oral form, making this route widely preferred in outpatient settings.
- The dominance of oral formulations is further supported by advancements in drug delivery technologies that improve bioavailability and reduce dosing frequency, enhancing patient adherence and treatment outcomes.
- On the other hand, the injectable segment is anticipated to be the fastest growing, driven by the rising adoption of biologics, gene therapies, and monoclonal antibodies for rare diseases. Injectable formulations are essential for therapies requiring targeted delivery, rapid onset, or complex molecular structures that cannot be administered orally.
Learn more about the key segments shaping this market
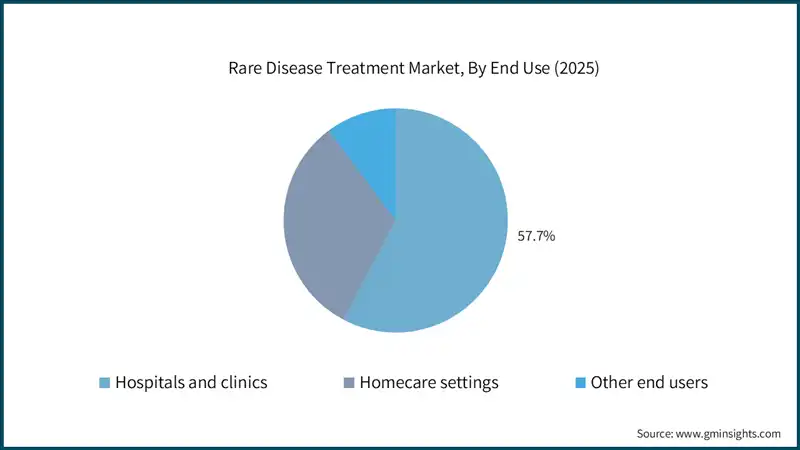
Based on the end use, the global rare disease treatment market is classified into hospitals and clinics, homecare settings, and other end users. The hospitals and clinics segment held a market share of 57.7% in 2025 and is projected to grow at a CAGR of 11.7% during the analysis period.
- Hospitals remain the primary centers for rare disease treatment due to their advanced infrastructure, specialized diagnostic capabilities, and access to complex therapies such as gene and cell treatments.
- Their dominance is supported by the presence of multidisciplinary teams, including geneticists, immunologists, and rare disease specialists, enabling comprehensive care and management of high-risk patients.
- Clinics play an important role in early diagnosis and ongoing management of rare diseases, offering genetic counseling, routine monitoring, and administration of certain oral and injectable therapies in outpatient settings.
Looking for region specific data?
North America Rare Disease Treatment Market
The North America market dominated the global market with a market share of 41.1% in 2025.
- North America holds a dominant market share position in the global market owing to the advanced healthcare infrastructure, strong and supportive regulatory approvals for orphan drugs, and high adoption of innovative therapies that include gene and cell treatments.
- The region benefits from early adoption of precision medicine, robust clinical trial networks, and significant investments in biotechnology and research and development.
- Additionally, the growing prevalence of rare genetic disorders, favorable reimbursement policies, and active patient advocacy groups further support strong market demand.
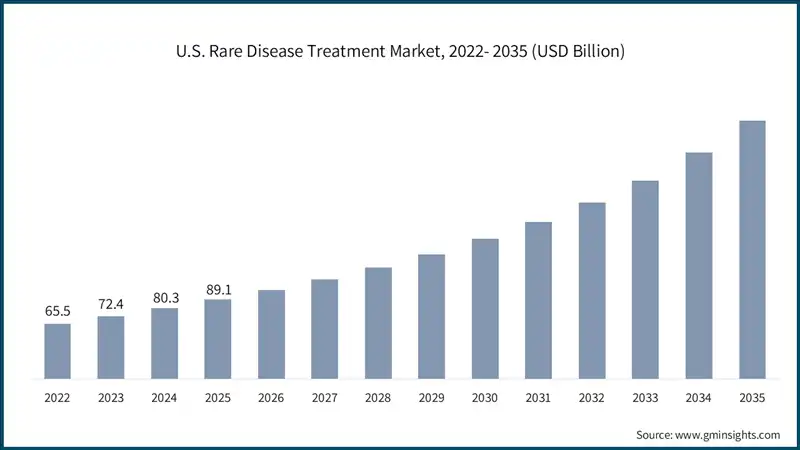
The U.S. market was valued at USD 65.5 billion and USD 72.4 billion in 2022 and 2023, respectively. The market size reached USD 89.1 billion in 2025, growing from USD 80.3 billion in 2024.
- The U.S. dominates the North America market, due to the highest number of orphan drug approvals and widespread adoption of advanced therapies.
- Moreover, a high number of rare diseases and people being affected further stimulate the market demand.
- For instance, according to the FDA, over 7,000 rare diseases affect more than 30 million people in the country, stimulating demand for specialized treatments and personalized care solutions.
Europe Rare Disease Treatment Market
Europe market accounted for USD 67.5 billion in 2025 and is anticipated to show lucrative growth over the forecast period.
- The Europe market is stimulated by high prevalence of rare disorders, strong government support for orphan drug development, and collaborative research initiatives across the region.
- According to European Commission, between 27 and 36 million people live with a rare disease, stimulating demand.
- The region benefits from a well-established network of academic institutions, biotech firms, and regulatory bodies, accelerating innovation and patient access to advanced therapies.
- Moreover, collaborative research ecosystems and early adoption of advanced treatment strengthen Europe’s position, while growing awareness campaigns improve diagnosis and treatment uptake.
Germany dominates the Europe market, showcasing strong growth potential.
- Germany holds a high market share due to its advanced healthcare infrastructure, strong clinical research ecosystem, and early adoption of gene and biologic therapies.
- The country has one of the largest rare disease patient registries in Europe, supporting clinical trials and personalized treatment programs.
- Moreover, Germany is home to several leading pharmaceutical and biotech companies actively engaged in orphan drug development, maintaining its position as a key innovation hub in the region.
Asia Pacific Rare Disease Treatment Market
The Asia Pacific market is anticipated to grow at the highest CAGR of 12.3% during the analysis timeframe.
- The Asia Pacific region is poised for high growth in the market, driven by rapidly expanding healthcare infrastructure, increasing genetic testing adoption, and rising prevalence of rare disorders.
- For example, according to the World Economic Forum, over 200 million people in Asia-Pacific are estimated to be living with rare diseases, with diagnosis rates improving due to government-led screening programs and patient advocacy initiatives.
- Additionally, growing investments in biotechnology and orphan drug development, coupled with supportive regulatory reforms in countries such as China and Japan, are creating significant opportunities for market expansion.
China rare disease treatment market is estimated to grow with a significant CAGR in the Asia Pacific market.
- China’s market growth is stimulated by a large patient base, rapid improvements in genetic testing, and government initiatives to include rare disease drugs in reimbursement lists.
- The country is witnessing increasing adoption of gene therapies and biologics, supported by local biotech innovation and partnerships with global pharmaceutical companies.
- Additionally, the establishment of national rare disease registries and specialized treatment centers stimulate patient access and clinical research, driving broader adoption of advanced therapies.
Latin American Rare Disease Treatment Market
Brazil leads the Latin American market, exhibiting remarkable growth during the analysis period.
- Brazil is experiencing an increase in rare disease diagnoses due to newborn screening programs and improved access to specialty care, which is stimulating demand for orphan drugs and advanced biologics.
- Additionally, government-backed initiatives to include rare disease treatments in public healthcare coverage and the expansion of specialized hospitals are supporting wider clinical adoption.
Middle East and Africa Rare Disease Treatment Market
Saudi Arabia market is expected to experience substantial growth in the Middle East and Africa market in 2025.
- Saudi Arabia’s market expansion is stimulated by increasing prevalence of genetic disorders, rising investments in healthcare infrastructure, and government-led rare disease programs under Vision 2030.
- The country is actively upgrading diagnostic capabilities and establishing specialized treatment centers, creating greater demand for orphan drugs and advanced therapies.
Rare Disease Treatment Market Share
The global market is moderately consolidated, with leading biopharmaceutical companies controlling a significant share while innovation thrives among specialized players. The top five companies Johnson & Johnson, Sanofi, AbbVie, F. Hoffmann-La Roche, and Novartis, collectively account for approximately 35% of the global market, leveraging strong research and development capabilities, advanced biologics platforms, and global commercialization networks. These companies continuously invest in gene therapies, monoclonal antibodies, and precision medicine solutions to address high unmet needs across rare cancers, immunological disorders, and genetic conditions.
The market landscape is further shaped by strategic acquisitions, licensing agreements, and collaborations, aimed at accelerating pipeline development and expanding access in emerging regions. Meanwhile, smaller biotech firms and niche players contribute by focusing on breakthrough therapies such as CRISPR-based gene editing and RNA-targeted drugs, fostering competitive differentiation. This dynamic environment supports rapid technological progress and sustained market growth, as demand for innovative rare disease treatments continues to rise globally.
Rare Disease Treatment Market Companies
Prominent players operating in the rare disease treatment industry are as mentioned below:
- AbbVie
- Alexion Pharmaceuticals
- Amgen
- AstraZeneca
- Baxter International
- Bayer
- Bristol-Myers Squibb
- Eli Lilly and Company
- F. Hoffmann La Roche
- GlaxoSmithKline
- Johnson & Johnson
- Merck & Co.
- Novartis
- Novo Nordisk
- Pfizer
- Sanofi
- Takeda Pharmaceutical
- Vertex Pharmaceutical
- Johnson & Johnson
Johnson & Johnson holds a strong position with ~10.8% in the market, driven by its advanced biologics and gene therapy platforms targeting rare cancers and immunological disorders. The company’s extensive R&D investments and global commercialization capabilities enable it to deliver innovative therapies and expand access across major markets.
AbbVie maintains a competitive edge through its robust pipeline in rare neurological and immunology conditions, supported by strategic acquisitions and partnerships. AbbVie’s focus on specialty drugs and precision medicine approaches reinforces its leadership in addressing high unmet needs in rare diseases.
F. Hoffmann-La Roche dominates rare oncology and hematology segments with cutting-edge biologics, targeted therapies, and personalized medicine solutions. Roche’s strong clinical research network and commitment to advancing rare cancer treatments position it as a key innovator in the global market.
Rare Disease Treatment Industry News
- In December 2025, Mirum Pharmaceuticals entered into a definitive agreement to acquire Bluejay Therapeutics for USD 250 million in cash and USD 370 million in stock, plus milestone payments. This acquisition strengthened Mirum’s rare liver disease portfolio, enhancing its pipeline and global leadership in rare disease treatments.
- In September 2025, SERB Pharmaceuticals completed its acquisition of Y-mAbs Therapeutics, making Y-mAbs a wholly owned subsidiary. The transaction included delisting Y-mAbs from Nasdaq and integrating its antibody-based oncology therapeutics into SERB’s portfolio.
- In June 2025, Sanofi acquired Blueprint Medicines, with additional milestone-based CVR payments. The deal included Ayvakit/Ayvakyt, the only approved therapy for systemic mastocytosis, and an early-stage immunology pipeline. The acquisition expanded Sanofi’s rare immunology portfolio.
- In April 2025, Merck entered into a definitive agreement to acquire SpringWorks Therapeutics. The acquisition added approved therapies for desmoid tumors and neurofibromatosis type 1-associated plexiform neurofibromas. The deal enhanced Merck’s rare oncology portfolio and increased its presence in the U.S. market.
The rare disease treatment market research report includes in-depth coverage of the industry with estimates and forecast in terms of revenue in USD Million from 2022 - 2035 for the following segments:
Market, By Drug Type
- Biologics
- Non-biologics
Market, By Therapeutic Area
- Cancer
- Blood-related disorders
- Central nervous system
- Respiratory disorders
- Musculoskeletal disorders
- Cardiovascular disorders
- Other therapeutic areas
Market, By Patient
- Adult
- Pediatric
Market, By Route of Administration
- Oral
- Injectable
- Other routes of administration
Market, By End Use
- Hospitals and clinics
- Homecare settings
- Other end users
The above information is provided for the following regions and countries:
- North America
- U.S.
- Canada
- Europe
- Germany
- UK
- France
- Spain
- Italy
- Netherlands
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East and Africa
- South Africa
- Saudi Arabia
- UAE
Frequently Asked Question(FAQ) :
Which therapeutic area leads the rare disease treatment industry?
The cancer segment led the market, generating USD 115.7 billion in revenue in 2025, due to the high prevalence of rare cancers and strong pipelines of targeted oncology and immunotherapy drugs.
Which drug type segment generated the highest revenue in 2025?
The biologics segment dominated the market with a 56.4% share in 2025, driven by the growing use of gene therapies, monoclonal antibodies, and enzyme replacement therapies for complex rare diseases.
What will be the market value of the rare disease treatment industry in 2026?
The market is projected to reach USD 265.5 billion in 2026, reflecting strong early growth driven by increasing orphan drug approvals.
What is the market size of the rare disease treatment market in 2025?
The global market for rare disease treatment was valued at USD 240.1 billion in 2025, driven by the high prevalence of rare genetic and metabolic disorders.
What is the projected value of the rare disease treatment market by 2035?
The market is expected to reach USD 726 billion by 2035, growing at a CAGR of 11.8% from 2026 to 2035, supported by advancements in gene and cell therapies.
Which end-use segment leads the rare disease treatment industry?
Hospitals and clinics dominated the market with a 57.7% share in 2025, due to advanced diagnostic capabilities, access to complex therapies, and presence of multidisciplinary rare disease care teams.
Which region leads the rare disease treatment market?
North America dominated the global market with a 41.1% share in 2025, supported by strong regulatory frameworks, high orphan drug approvals, and rapid adoption of gene and cell therapies.
Who are the key players in the rare disease treatment market?
Major players include Johnson & Johnson, Sanofi, AbbVie, F. Hoffmann-La Roche, Novartis, Pfizer, AstraZeneca, Vertex Pharmaceuticals, Takeda Pharmaceutical, and Alexion Pharmaceuticals.
Rare Disease Treatment Market Scope
Related Reports


