Home > Healthcare > Medical Devices > Diagnostic Devices > Point of Care Molecular Diagnostics Market
Point of Care Molecular Diagnostics Market Size
- Report ID: GMI2210
- Published Date: Jan 2023
- Report Format: PDF
Point of Care Molecular Diagnostics Market Size
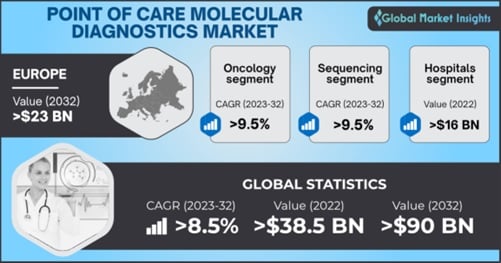
Point of Care Molecular Diagnostics Market size was valued at more than USD 38.5 billion in 2022 and is projected to grow substantially at over 8.5% growth rate from 2023 to 2032 owing to the increasing efforts for advancing diagnostic procedures.
R&D initiatives for improving diagnostic procedures for easy analysis of complex and severe diseases are foreseen to stimulate industry expansion. Moreover, the increasing number of favorable government programs supporting the development of molecular diagnostics owing to their medical, economic, and societal advantages are projected to augment point of care molecular diagnostics market revenues.
| Report Attribute | Details |
|---|---|
| Base Year: | 2022 |
| Point of Care Molecular Diagnostics Market Size in 2022: | USD 38.5 Billion |
| Forecast Period: | 2023 to 2032 |
| Forecast Period 2023 to 2032 CAGR: | 8.5% |
| 2032 Value Projection: | USD 90 Billion |
| Historical Data for: | 2018 to 2022 |
| No. of Pages: | 170 |
| Tables, Charts & Figures: | 174 |
| Segments covered: | Technology, Application, End-use, and Region |
| Growth Drivers: |
|
| Pitfalls & Challenges: |
|
Complicated regulatory processes may slow down market progress
The business growth is hampered by complicated regulatory procedures such as pre- and post-market clearances and multiple clinical testing. For instance, the Clinical Laboratory Improvement Amendments (CLIA) rules mandate that the QC (Quality Control) for illness testing must be completed in accordance with the manufacturer’s instructions. In the absence of a specification from the manufacturer, the testing facility must establish a policy that adheres to acceptable laboratory procedures. However, with the growing awareness about the product’s benefits among buyers, the government may relax the requirements for adhering to strict laws, which is foreseen to benefit the market outlook.