Home > Healthcare > Medical Devices > Diagnostic Devices > Point of Care Molecular Diagnostics Market
Point of Care Molecular Diagnostics Market Analysis
- Report ID: GMI2210
- Published Date: Jan 2023
- Report Format: PDF
Point of Care Molecular Diagnostics Market Analysis
By technology, the point of care molecular diagnostics market share from the sequencing segment is anticipated to record growth at more than 9.5% CAGR over 2023-2032. The development of higher-quality genetic sequencing is favorably influencing segment development. Additionally, favorable government policies promoting the adoption of modern technology are expected to bolster segment progress.
In terms of end-use, the point of care molecular diagnostics market is segmented into hospitals, clinics, diagnostic centers, and others. The hospitals segment accounted for a valuation of over USD 16 billion in 2022, the report claims. Easy access to healthcare professionals and the surging availability of advanced medical equipment and treatments are the major factors estimated to propel industry outlook. In November 2021, healthcare giant Roche launched cobas® 5800 System, a molecular laboratory device that assists hospitals and laboratories in addressing challenges brought on by increasing patient testing and reimbursement complexities.
By application, the point of care molecular diagnostics market from oncology segment is projected to grow at over 9.5% CAGR through 2032 attributed to the high prevalence of cancer cases and rising healthcare expenditure. According to the National Center for Biotechnology, China was estimated to have 4,820,000 new cancer cases in 2022. Increasing regulatory approvals for oncology PoC molecular diagnostic products further is set to contribute to segment growth. In August 2022, Canhelp Genomics, a Chinese genetic diagnostics business, attained National Medical Products Administration (NMPA) approval for its Canhelp-Origin pan-cancer test and companion analytic software.
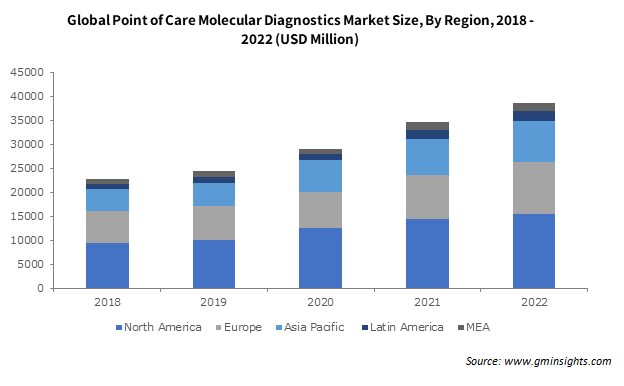
Europe point of care molecular diagnostics market valuation is estimated to reach over USD 23 billion by 2032 owing to the proliferation of molecular technology for the rising need for affordable, quick, and portable molecular diagnostics. Additionally, the emergence of several significant market participants is expected to fuel the regional market revenue. For instance, in March 2022, Sense Biodetection (Sense), a pioneer in molecular diagnostics pioneer received the CE Marking in Europe, for its Veros COVID-19, a fully integrated, easy-to-use molecular diagnostic test that offers laboratory-quality results in 15 minutes.

