Home > Healthcare > Pharmaceuticals > Active Pharmaceutical Ingredients > Phenylephrine Hydrochloride Market
Phenylephrine Hydrochloride Market Analysis
- Report ID: GMI6848
- Published Date: Oct 2023
- Report Format: PDF
Phenylephrine Hydrochloride Market Analysis
Based on application, the global phenylephrine hydrochloride is segmented into nasal decongestion, cold, hypotension, allergic reaction, eye diseases, and other applications. The nasal decongestion segment dominated the global phenylephrine hydrochloride market with market share of 29.5% in 2022. Phenylephrine HCl (Phenylephrine Hydrochloride) API is a vital component in nasal decongestant medications, serving as the active ingredient that provides relief from nasal congestion. This pharmaceutical compound functions by constricting the blood vessels in the nasal passages, effectively reducing the swelling and congestion associated with various respiratory conditions, including the common cold, allergies, sinusitis, and hay fever.
Further, phenylephrine HCl is formulated into a range of nasal decongestant products, including nasal sprays, nasal drops, and oral tablets. These formulations are readily available over-the-counter (OTC) and by prescription, catering to individuals seeking quick and effective relief from nasal congestion

By mode, the phenylephrine hydrochloride market is segmented into in-house manufacturing and contract manufacturing. The in-house manufacturing segment held significant market share of 72.2% in 2022 and is expected to witness growth at CAGR of 7.6% over the forecast timeframe. In-house manufacturing of phenylephrine hydrochloride PAI enables pharmaceutical companies to exert precise control over every aspect of the production process. It offers greater flexibility to pharmaceutical companies for customizing the production according to their specific requirements and market demands.
Additionally, for companies involved in pharmaceutical research and development (R&D), in-house manufacturing of APIs can enable smooth integration between R&D and production departments. This integration facilitates the transition from drug development to commercial production, reducing lead times and optimizing the workflow and development process. All these factors together contribute towards the high segmental progress.
By use, the phenylephrine hydrochloride market is segmented into human and veterinary. The human segment dominated the global market with highest market share of 75.5% in 2022. Phenylephrine HCl API is subject to strict quality and safety standards to ensure its suitability for human use. It is typically produced in accordance with good manufacturing practices (GMP) and undergoes rigorous testing to meet pharmaceutical quality requirements. As with any medication, it should be used according to healthcare provider recommendations, or the instructions provided on the product label to ensure safe and effective use for human health.
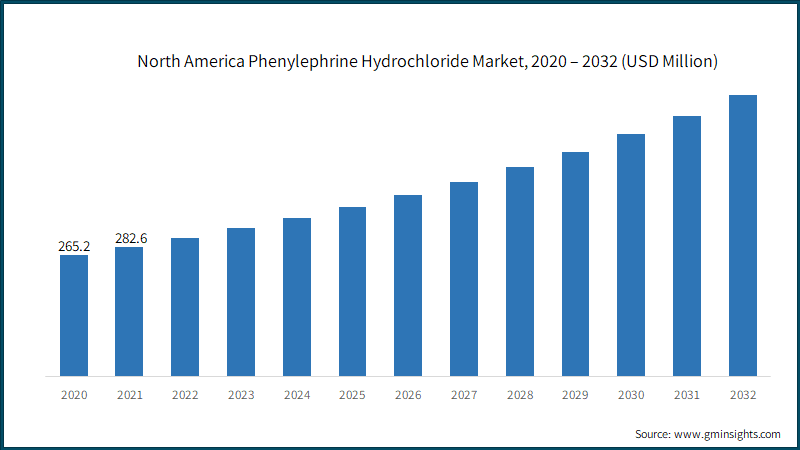
The North America dominated the phenylephrine hydrochloride market with a business share of 38.9% in 2022. Phenylephrine HCl (Phenylephrine Hydrochloride) API in the North American region holds significant importance in the pharmaceutical sector, primarily due to its key role in the production of nasal decongestant medications. North America is home to a diverse array of pharmaceutical manufacturers, both domestic and international, that produce Phenylephrine HCl API. These manufacturers operate within a regulatory framework overseen by agencies like the U.S. Food and Drug Administration (FDA) in the United States and Health Canada, ensuring that the manufacturing processes meet stringent quality and safety standards. As North America continues to lead in pharmaceutical innovation and market penetration, the high growth of the market is poised to continue over the analysis period.

