Home > Healthcare > Healthcare IT > Surveillance Solutions > Pharmacovigilance Outsourcing Market
Pharmacovigilance Outsourcing Market Size
- Report ID: GMI3030
- Published Date: Mar 2020
- Report Format: PDF
Pharmacovigilance Outsourcing Market Size
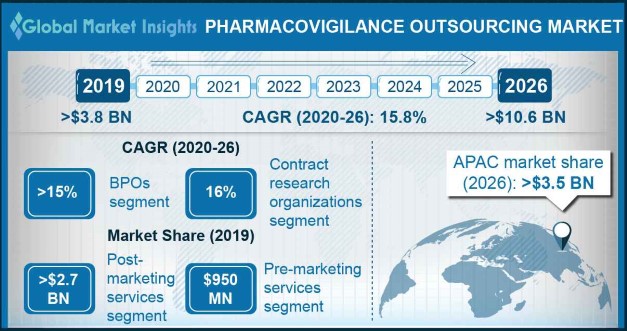
Pharmacovigilance Outsourcing Market size exceeded USD 3.8 billion in 2019 and is set to grow at around 15.8% CAGR between 2020 and 2026.
Pharmacovigilance (PV) can be defined as science and activities pertaining to the detection, understanding, assessment and prevention of drug-related problems. It plays a significant role in developing the healthcare system by assessing and monitoring the adverse drug interactions and their effects on human. The number of adverse drug reactions (ADRs) reported during the last few years has increased to a great extent and requires high level of expertise in pharmacovigilance to rapidly detect drug risks and defend the product against recalls.
| Report Attribute | Details |
|---|---|
| Base Year: | 2019 |
| Pharmacovigilance Outsourcing Market Size in 2019: | USD 3.8 Billion |
| Forecast Period: | 2020 to 2026 |
| Forecast Period 2020 to 2026 CAGR: | 15.8% |
| 2026 Value Projection: | USD 10.6 Billion |
| Historical Data for: | 2015 to 2019 |
| No. of Pages: | 225 |
| Tables, Charts & Figures: | 207 |
| Segments covered: | Service, Service Provider and Region |
| Growth Drivers: |
|
| Pitfalls & Challenges: |
|
Pharmacovigilance outsourcing refers to transfer of drug safety processes and functions to a third-party provider. These services are majorly outsourced by small and mid-size pharmaceutical and biotechnology companies with an aim to save costs and focus on company’s core activities. Some of the common PV activities that are generally outsourced include collecting ADR information, case processing activities, development of risk management plans & risk evaluation mitigation strategy as well as creating & submitting aggregate and expedited PV reports. Improved focus of companies on outsourcing certain drug safety processes to third party to lower costs and improve efficiency will escalate the market expansion.