Home > Healthcare > Healthcare IT > Surveillance Solutions > Pharmacovigilance Outsourcing Market
Pharmacovigilance Outsourcing Market Analysis
- Report ID: GMI3030
- Published Date: Mar 2020
- Report Format: PDF
Pharmacovigilance Outsourcing Market Analysis
Adverse drug reactions associated with pharmaceutical products used in prevention and treatment of various diseases will serve to be one of the major factors fostering the market growth. ADR monitoring is required for each drug throughout its life cycle, from drug development process including pre-marketing, early stages of drug design and clinical trials to post-marketing surveillance. Development in drug discovery process has led to availability of multiple new drugs in the market. Compliance of these drugs with the safety parameters laid by the regulatory authorities will upsurge the need for pharmacovigilance services.
Pharmaceutical and biopharmaceutical companies rely on pharmacovigilance activities that are performed in-house or are outsourced. Challenges in setting up in-house PV department such as high costs to maintain required levels of compliance, infrastructure and availability of qualified and trained in-house resources will lead to rise in outsourcing trends. Increasing number of pharmaceutical companies outsourcing their PV activities to service providers such as contract research organizations (CROs) and business process outsourcing (BPOs) will further spur the pharmacovigilance outsourcing market share.
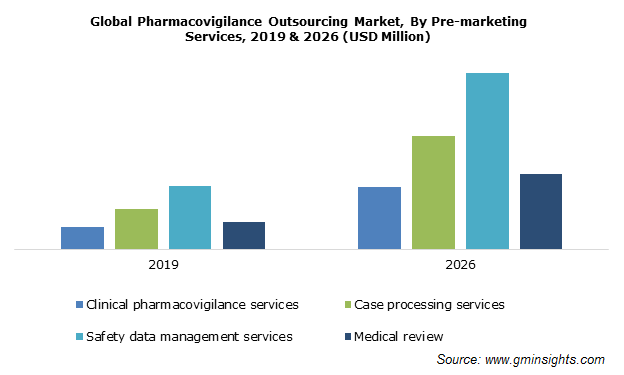
The pre-marketing services cover clinical pharmacovigilance services, case processing services, safety data management services and medical review. Post marketing services include knowledge process outsourcing services and IT solutions & services.
The pre-marketing services segment was valued around USD 950 million in 2019 and is estimated to grow significantly during the forecast period. Increasing focus of companies on product monitoring, clinical operations, regulatory affairs, statistical analysis, quality assurance, evaluation and drug approvals with necessary clearances and documentation will favor the pharmacovigilance outsourcing industry growth.
Pre-marketing surveillance involves data collection regarding adverse drug reactions from the pre-clinical screening to phases III clinical trials. Linking pre-marketing with human safety information is one of the emerging trends in pharmacovigilance outsourcing. Moreover, rising investments in R&D activities for developing computational approaches to predict potential ADRs using pre-clinical characteristics of the compounds or post-screening data will thus, prove beneficial for the market progress.
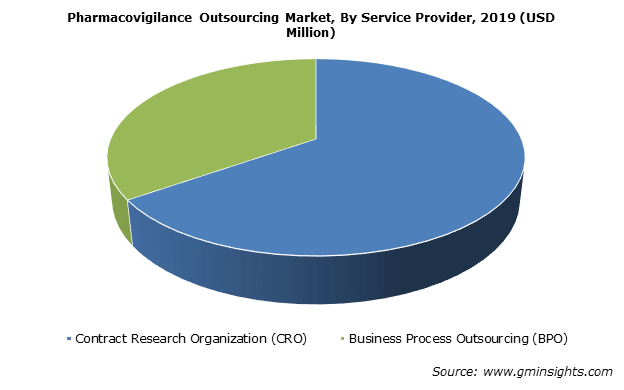
The contract research organizations segment of pharmacovigilance outsourcing market is anticipated to witness 16% CAGR over 2020 to 2026. Segment growth is attributed to dependence of pharma and biotech companies on CROs to carry out pharmacovigilance activities.
The small and medium-size pharmaceutical/biotechnology companies generally do not have a separate facility for performing PV activities to ensure drug safety. Outsourcing PV activities to CROs, reduce the cost of setting up an entire pharmacovigilance unit and enable time management for the small-size companies. Growing preference of drug safety teams towards outsourcing case management activities to CROs will thus, boost the market growth.
Asia Pacific industry is poised to exceed USD 3.5 billion revenue by 2026. Increasing volume of clinical trials being conducted in the Asian countries will serve to be a major impact rendering factor in the regional market growth. Developing countries such as China and India are the most favored destinations for PV outsourcing owing to availability of high skill set at lower costs. Moreover, availability of large pool of talented medical, paramedical and non-medical professionals involved in the PV process coupled with presence of refined PV systems in the region will further accelerate the market progression.