Summary
Table of Content

Molecular Quality Controls Market
Get a free sample of this report
Form submitted successfully!
Error submitting form. Please try again.
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!

Request Sectional Data
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!
Form submitted successfully!
Error submitting form. Please try again.
Molecular Quality Controls Market Size
The global molecular quality controls market size was estimated at USD 215.2 million in 2024. The market is expected to grow from USD 228 million in 2025 to USD 413.5 million by 2034 at a CAGR of 6.8%. Molecular quality controls are reference materials which are used in molecular diagnostic tests to ensure the accurateness, consistency, and reliability of results. These controls helps laboratories to monitor assay performance, to detect errors, and to comply with the regulatory standards by proving the precision of molecular testing methods.

To get key market trends
The global market is experiencing significant growth, driven by an increasing cancer and infectious diseases cases. For instance, according to the estimates from the World Health Organization (WHO), in 2022 there were approximately 20 million cancer cases with 9.7 million cancer deaths reported worldwide. It is estimated that 53.5 million people were cancer survivors within five years of getting diagnosed with the illness.
Molecular Quality Controls Market Report Attributes
| Key Takeaway | Details |
|---|---|
| Market Size & Growth | |
| Base Year | 2024 |
| Market Size in 2024 | USD 215.2 Million |
| Forecast Period 2025 – 2034 CAGR | 6.8% |
| Market Size in 2034 | USD 413.5 Million |
| Key Market Trends | |
| Growth Drivers |
|
| Pitfalls & Challenges |
|
What are the growth opportunities in this market?
It is said that every one in five people across the globe will be diagnosed with cancer at some point in their life, while one in nine men and one in twelve women will die because of it. These statistics represent the rising demand for molecular quality controls in oncology, which is estimated to reach USD 866.1 billion by 2034, diagnostics, to ensure the accurateness and consistency of cancer detection tests. Molecular diagnostics play a vital role in early cancer detection, treatment selection, and monitoring, thus, the need for high-quality controls to validate assay performance is growing.
Additionally, the growing demand for accurate diagnostic testing is one of the driver, which drive the growth of molecular quality control market. As healthcare industry grows, there is a rising need for precise and reliable molecular diagnostics to monitor medical decisions, particularly in infectious disease detection, cancer, and genetic testing, among others. Thus, the demand directly impacts the need for strong molecular quality control systems to ensure the accuracy and reliability of polymerase chain reaction, next-generation sequencing, and other nucleic acid-based tests.
Further, the growing prevalence of infectious diseases includes HIV, Hepatitis B (HBV), Hepatitis C (HCV), influenza (Flu), among others and genetic disorders has surged globally, escalating the need for continuous and accurate molecular diagnostics. For instance, according to World Health Organization, in 2023 39.9 million individuals were living with HIV. An estimated 0.6% of adults aged 15-49 years, globally lived with HIV, thus this highlights the growing need for molecular testing and the necessity of stringent quality control measures. Moreover, growing adoption of molecular diagnostics and technological advancements in molecular diagnostics, are further contributing to the growth of the market.
Molecular Quality Controls Market Trends
- Technological innovation in molecular diagnostics have transformed the molecular quality control market, by enhancing the accuracy, effectiveness and consistency of diagnostic testing. These advancements ensures that molecular assays meet regulatory and performance standards which ultimately improves patient outcomes.
- Digital PCR (dPCR) has emerged as a vital technology for absolute nucleic acid quantification. Digital PCR partitions, the sample into thousands of separate reactions, which allows for accurate and highly sensitive detection of low-abundance targets. Thus, this significant applications are used in oncology, infectious diseases, and genetic testing.
- For example, in September 2024, Qiagen launched QIAcuityDx Digital PCR System, a pivotal addition to its digital PCR portfolio, which expanded into clinical diagnostics.
- Additionally, next-generation sequencing (NGS) has transformed molecular diagnostics by allowing the rapid sequencing of entire genomes, transcriptomes, and metagenomes. The rising adoption of NGS in clinical diagnostics, specifically in cancers, infectious disease screening, and personalized medicine, have driven the demand for advanced molecular quality controls.
- Further, AI integration into molecular diagnostics have improved data analysis and projecting capabilities. For instance, AI driven tools helps in analyzing complex datasets, identify patterns, and predict disease outcomes, thereby expanding the accuracy of diagnostic testing. Thus, these innovations necessitate the development of robust quality control measures to ensure that AI algorithms produce reliable and fair results in clinical applications.
- Moreover, isothermal amplification methods, such as loop-mediated isothermal amplification (LAMP), have achieved popularity due to their rapid, efficient, and low-cost nucleic acid detection capabilities. For instance, a study also showed that testing with LAMP is inherently simpler and more portable than conventional RT-qPCR.
- Thus, this technology is widely used to detect diseases such as COVID-19, tuberculosis, and malaria because it is fast, efficient, and works without special lab equipment. To make sure these tests are accurate and reliable, molecular quality controls help check for errors, reduce false results, and maintain consistent performance in different conditions.
Molecular Quality Controls Market Analysis

Learn more about the key segments shaping this market
Based on product, the market is segmented into independent controls and instrument-specific controls. Further, independent controls are bifurcated into polymerase chain reaction, DNA sequencing and NGS, and other instrument-specific controls. The market was estimated at USD 203.5 million in 2023. The independent controls held revenue of USD 116.7 million in 2024 and the segment is poised for significant growth at a CAGR of 6.7% during the forecast period.
- Independent controls are manufactured separately from the test kits, which ensures an objective for evaluation of molecular assay performance. Thus, instruments-specific controls, are designed to work optimally with a particular assay. For example, SeraCare’s AccuPlex recombinant viral controls are widely used to assess the accuracy of COVID-19 diagnostic assays.
- Additionally, independent controls also help to identify the issues that might not be detected with kit-specific controls, such as batch-to-batch variations, reagent stability problems or instruments malfunctions. By repeatedly using independent controls, laboratories can detect deviations early and take corrective action before inaccurate results impact patient care.
- Further, independent quality controls also support external quality assessment (EQA) and proficiency testing programs, which are an essential for meeting regulatory requirements. For instance, Microbiologics' Helix Elite molecular standards serve as controls for developing, validating, and monitoring molecular assays and instruments, ensuring regulatory compliance and test reliability. They are available in two distinct formats.
- This controls enable long-term assay performance monitoring, ensuring consistency and accuracy in molecular diagnostics despite changes in reagents, instruments, or personnel, thereby driving market growth.
- Moreover, independent controls allow long-term assay performance monitoring, ensuring consistency, and accurateness in molecular diagnostics despite changes in reagents, instruments, thus, propelling the market growth.
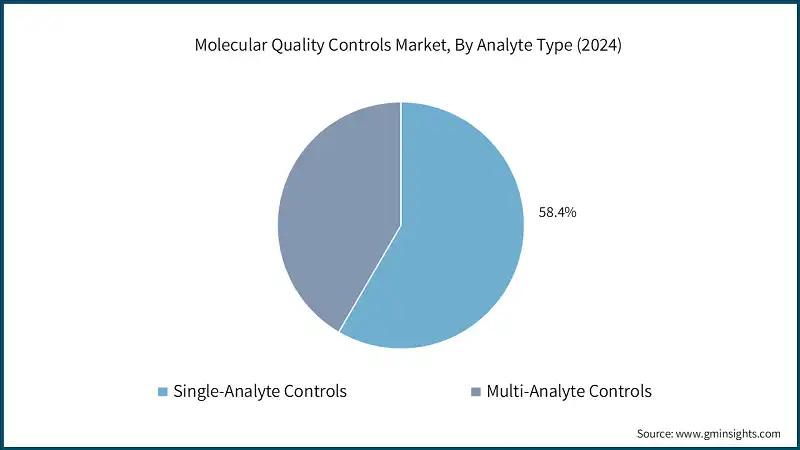
Learn more about the key segments shaping this market
Based on analyte type, the molecular quality controls market is bifurcated into single-analyte controls and multi-analyte controls. The single-analyte controls segment accounted for a revenue share of 58.4% and with revenue of USD 125.7 million in 2024.
- Single-analyte controls allow precise assessment of individual target analytes eliminating cross-reactivity risks which is associated with multi-analyte controls. These specificity ensure true monitoring of assay performance for particular biomarkers or genetic sequences.
- For example, ZeptoMetrix provides molecular quality controls that can be used across various molecular testing platforms and assays, which available in different formats including single-analyte controls.
- The single-analyte controls allows laboratories to choose controls specific to assays, which minimizes unnecessary complications. This customization is specifically valuable for labs conducting particular tests.
- Further, these analyte are made for high stability, which ensures constant performance over time and also minimizes assay variability. Thus, this reliability is vital for maintaining quality assurance in molecular testing, escalating the market growth.
Based on application, the molecular quality controls market is bifurcated into infectious disease diagnostics, oncology testing, genetic testing, and other applications. The infectious disease diagnostics segment accounted for a 48.2% market share in 2024.
- The increasing global prevalence of infectious disease such as respiratory infections, sexually transmitted infections (STIs), HIV/AIDS, Hepatitis B and C, Ebola, among others, are driving the demand for molecular quality controls.
- For instance, according to a study, globally, the highest incidence rates of URIs were seen in children younger than 2 years in 2021, and the largest number of episodes was in children aged 5–9 years.
- Molecular diagnostics can detect pathogens at an early stage of infection, even before symptoms appear. Thus, molecular quality controls confirms that these rapid assays remain reliable over the time.
- Further, molecular quality control serve as an early warning system for potential assay failures, reagent degradation, or cross-contamination in the laboratory. Thus, by detecting errors at an early stage, molecular quality control helps to prevent inaccurate results that could comprise clinical decisions.
- For instance, molecular quality control are used in tuberculosis PCR assays which are used to identify potential reagent detection, which ensures the reliability of test results. Thus, ability this ability to identify errors before they affect patients health, improves overall lab effectiveness and patient safety.
Based on end use, the molecular quality controls market is bifurcated into hospitals, diagnostic laboratories, academic and research institutes, contract research organizations, and other end users. The diagnostic laboratories segment accounted for a 34.5% market share in 2024.
- Diagnostic laboratories encounter a greater number of patients samples, undergoing complex molecular diagnostic testing which include infectious diseases detection, oncology screening and genetic disorder analysis.
- Diagnostic laboratories capability to address larger volume of molecular tests in both routine as well as in specialized diagnostics making them a key contributor for adoption of molecular quality controls, thereby propelling the market growth.
- Moreover, diagnostic laboratories encompass large number of skilled professionals such as molecular biologists, pathologists, and laboratory technicians ensures that accurate test execution and interpretation, making them a preferred choice for clinical and research based molecular testing.
- Furthermore, these facilities are well equipped with advanced molecular diagnostic instruments, such as PCR systems, next generation sequencing and automated sample processing units, along with dedicated quality controls programs thereby boosting the segmental growth.

Looking for region specific data?
The North America molecular quality controls market is accounted for USD 87.1 million revenues in 2024 forecasted to reach USD 164.7 million by 2034. The U.S. dominated the North America market with the largest revenue of USD 75.5 million in 2023.
- The increasing incidence of infectious diseases and cancer in the U.S. drives demand for molecular quality controls. For instance, according to the National Cancer Institute, in 2024, it is estimated that 2,001,140 new cases of cancer diagnosed in the U.S., with 611,720 fatalities resulting from the disease.
- In addition, the U.S. Food and Drug Administration (FDA) has implemented rigorous standards for molecular quality controls, confirming the accuracy and reliability of molecular diagnostic tests across various applications, including infectious disease detection, oncology, and genetic testing.
Europe: The molecular quality controls market in UK is expected to experience significant and promising growth from 2025 to 2034.
- The UK government is heavily investing in molecular quality control services, to enhance diagnostic accuracy, standardization, and regulatory compliance.
- For instance, in 2022, as per the data from University of Oxford, the Cancer Research UK had invested around EUR 11 million in the cancer science research in Oxford.
- Thus, these investment is anticipated to stimulate groundbreaking innovations in molecular quality controls, improving assay reliability, and strengthen the regulatory framework, accelerating market growth in the country.
Asia Pacific: Japan molecular quality controls industry is anticipated to witness lucrative growth between 2025 – 2034.
- According to the World Health Organization (WHO), in 2021, Japan's elderly population, aged 65 and above, comprised 29.1% of the total population. This demographic shift has led to a rise in age-related diseases, with cancer as the leading cause of death, accounting for 27% of all fatalities in Japan, as reported by the National Cancer Center Japan in 2022. This increasing disease burden has surged the demand for effective therapies, escalating the growth of the market.
- Thus, as the population ages in the country so does the chances of cancer, thereby fostering the market growth in the country.
Middle East and Africa: The molecular quality controls market in Saudi Arabia is expected to experience significant and promising growth from 2025 to 2034.
- Saudi Arabia is also experiencing a shift in population demographics. For instance, the number of people aged 60+ is expected to increase five times from 2 million (5.9% of the total population) in 2020, reaching 10.5 million by 2050.
- Such high trends in the aging population are expected to increase the demand for molecular quality controls in coming years.
- Further, Saudi Arabia’s advance healthcare infrastructure and rising investment in advanced healthcare technologies create opening for the development and adoption of advanced molecular quality controls tailored to the country’s patient specific needs.
Molecular Quality Controls Market Share
The top 4 market players of the market account for approximately 45% of the market share which includes companies such as Bio-Rad Laboratories, Bio-Techne Corporation, Qiagen, and Thermo Fisher Scientific, among others. With every firm introducing new and advanced technologies, modernization is extremely important.
Moreover, strategic partnerships with companies, research institutes, and government agencies play a primary role in advancing the development of products and getting the necessary permits. The enhancement of public awareness about chronic conditions such cancer, infectious diseases and its health impact through the social media platform, will encourage more individuals to seek the treatment, assisting market players to strengthen their position in this growing sector.
Molecular Quality Controls Market Companies
Some of the eminent market participants operating in the molecular quality controls industry include:
- Anchor Molecular
- Bio-Rad Laboratories
- Bio-Techne Corporation
- Fortress Diagnostics
- Microbiologics
- Microbix Biosystems
- Maine Molecular Quality Controls
- Quidelortho Corporation
- Qiagen
- Randox Laboratories
- Seegene
- Thermo Fisher Scientific
- Zeptometrix
- Bio-Rad Laboratories has robust geographical presence which enables it to enhance its market reach. Bio-Rad Laboratories operates in more than 35 countries thus comprising of a strong distribution network.
- Thermo Fisher Scientific has strong global workforce of approximately 122,000 employees, which enables the company to drive innovation, and deliver high-quality solutions.
Molecular Quality Controls Industry News:
- In August 2021, Bio-Rad Laboratories launched the SARS-CoV-2 S Gene Alpha, Beta, Gamma and Epsilon Variant Controls as part of its Exact Diagnostics line of molecular quality control products for research testing. This launch strengthened Bio Rad’s position in molecular quality control market.
- In February 2022, ZeptoMetrix launched the SARS-CoV-2 Omicron Control. This molecular quality control is one more addition to the company’s expansive line of infectious disease quality controls and made available for pre-order. This launched expanded ZeptpMetrix product portfolio and strengthened its reputation for innovation in infectious disease diagnostics.
The molecular quality controls market research report includes an in-depth coverage of the industry with estimates and forecast in terms of revenue in USD Million from 2021 – 2034 for the following segments:
Market, By Product
- Independent controls
- Instrument-specific controls
- Polymerase chain reaction
- DNA sequencing and NGS
- Other instrument-specific controls
Market, By Analyte Type
- Single-analyte controls
- Multi-analyte controls
Market, By Application
- Infectious disease diagnostics
- Oncology testing
- Genetic testing
- Other applications
Market, By End Use
- Hospitals
- Diagnostic laboratories
- Academic and research institutes
- Contract research organizations
- Other end use
The above information is provided for the following regions and countries:
- North America
- U.S.
- Canada
- Europe
- Germany
- UK
- France
- Spain
- Italy
- Netherlands
- Asia Pacific
- China
- Japan
- India
- Australia
- South Korea
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East and Africa
- South Africa
- Saudi Arabia
- UAE
Frequently Asked Question(FAQ) :
What was the value of the North America molecular quality controls market in 2024?
The North America market was valued at USD 87.1 million in 2024.
Who are some of the prominent players in the molecular quality controls industry?
Key players in the molecular quality controls industry include Anchor Molecular, Bio-Rad Laboratories, Bio-Techne Corporation, Fortress Diagnostics, Microbiologics, Microbix Biosystems, Maine Molecular Quality Controls, Quidelortho Corporation, Qiagen, Randox Laboratories, Seegene, Thermo Fisher Scientific, and Zeptometrix.
How big is the global molecular quality controls market?
The global market size for molecular quality controls was estimated at USD 215.2 million in 2024 and is expected to grow at a 6.8% CAGR from 2025 to 2034.
What is the expected growth rate of the independent controls segment?
The independent controls segment is poised for significant growth at a CAGR of 6.7% during the forecast period.
How much revenue did the independent controls segment generate in 2024?
The independent controls segment held revenue of USD 116.7 million in 2024.
Molecular Quality Controls Market Scope
Related Reports


