Home > Healthcare > Healthcare IT > Surveillance Solutions > Medical Devices Vigilance Market
Medical Devices Vigilance Market Size
- Report ID: GMI3140
- Published Date: Feb 2019
- Report Format: PDF
Medical Devices Vigilance Market Size
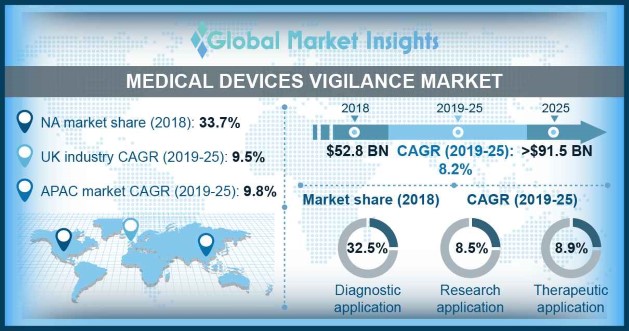
Medical devices vigilance market size was valued at USD 52.8 billion in 2018 and is expected to witness 8.2% CAGR from 2019 to 2025.
Surge in the number of medical device recalls due to safety issues is the key factor driving the global market growth during the forecast period. According to FDA, in 2015, around 1.4 million adverse events reported in the U.S. agency were sent to the FDA through medical device reporting system. Growing awareness among people pertaining to availability of medical device vigilance software and reporting of adverse events will positively influence medical devices vigilance industry growth over the analysis timeframe.
| Report Attribute | Details |
|---|---|
| Base Year: | 2018 |
| Medical Devices Vigilance Market Size in 2018: | 52.8 Billion (USD) |
| Forecast Period: | 2019 to 2025 |
| Forecast Period 2019 to 2025 CAGR: | 8.2% |
| 2025 Value Projection: | 91.5 Billion (USD) |
| Historical Data for: | 2014 to 2018 |
| No. of Pages: | 145 |
| Tables, Charts & Figures: | 202 |
| Segments covered: | Delivery Mode, Application, End-user and Region |
| Growth Drivers: |
|
| Pitfalls & Challenges: |
|
Additionally, favourable government scenario pertaining to medical devices vigilance to ensure patient safety will serve to be another impact rendering factor that will foster the business growth. Increasing burden on medical devices manufacturers to produce safe medical equipment as well as stringent safety regulations laid by regulatory authorities pertaining to pre- and post-commercialization of medical devices will further spur the market size. However, negligence of manufacturing companies towards product safety may restrain medical devices vigilance industry growth over the coming years.