Home > Healthcare > Medical Services > Lab Services > Laboratory Proficiency Testing Market
Laboratory Proficiency Testing Market Analysis
- Report ID: GMI3571
- Published Date: May 2023
- Report Format: PDF
Laboratory Proficiency Testing Market Analysis
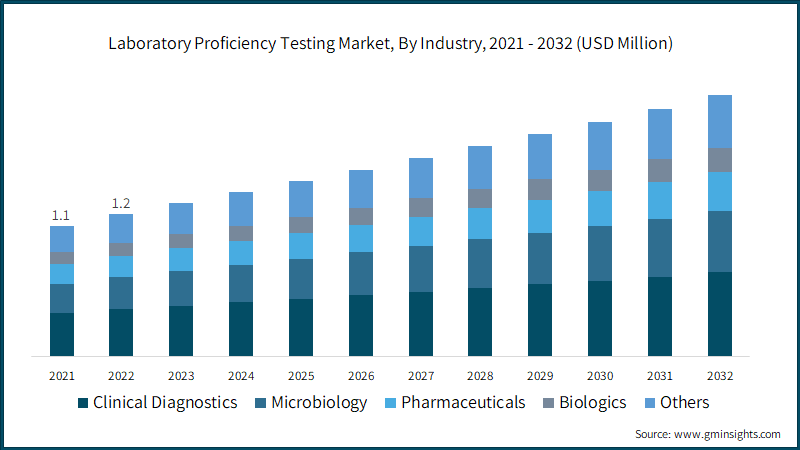
Based on industry, the market is categorized into clinical diagnostics, microbiology, pharmaceuticals, biologics, and others. The clinical diagnostics segment accounted for largest revenue share in 2022 and is predicted to reach around USD 723 million in 2032. The high revenue share is primarily attributed to increasing trend towards early disease diagnosis, growing need for development of complex diagnostic tests, among others. Further, use of proficiency testing in clinical diagnostics makes quality management a key pointer, thereby, allowing test reproducibility and improving the efficiency as well as accuracy of tests.
Based on technology, the laboratory proficiency testing market is segmented into polymerase chain reaction, cell culture, spectrometry, chromatography, immunoassays, and others. The cell culture segment accounted for major revenue share and was valued at around USD 355 million in 2022. Increasing use of cell culture in pharmaceutical industry owing to numerous benefits including vaccines and cell culture-based products will fuel the segmental growth. Additionally, adoption of cell culture-based products such as monoclonal antibodies, further boosted the adoption of cell culture tests to maximize the output from microbial strain cultures.

Based on end-use, the laboratory proficiency testing market is bifurcated into hospitals, diagnostic laboratories, contract research organizations, academic research, and pharmaceutical & biotechnology companies. The pharmaceutical & biotechnology companies segment held the significant revenue share in 2022 and is anticipated to witness a CAGR of 6.4% throughout the forecast period. Pharmaceutical companies play a critical role in research, development, and manufacturing, particularly in areas such as vaccines and blood and tissue-related research. These organizations must adhere to stringent sterility and quality standards for both raw materials and finished products. To ensure accurate results and laboratory efficiency, proficiency testing services are employed in these firms. These services enable monitoring and modification of flawed processes, leading to improved overall functioning and product quality in line with regulatory guidelines.

North America held a dominant market share in the global laboratory proficiency testing market and is expected to witness at 5.5% CAGR through 2032. This high market growth is owing to several factors such as increasing regulatory compliance, technological advancements, and industrial collaboration among others. Additionally, presence of key market players, an increase in the number of chronic diseases, and rising awareness regarding several clinical benefits offered by laboratory proficiency testing is expected to boost the market growth. Regulatory agencies, such as the FDA and Health Canada, have implemented stringent quality standards and regulations for laboratories. Moreover, laboratories in North America are adopting proficiency testing to ensure compliance with these regulations and demonstrate their commitment to quality. Therefore, technological advancements, government support & initiatives and aforementioned factors are expected to augment the regional growth in the near future.