Home > Healthcare > Medical Devices > Diagnostic Devices > Influenza Diagnostic Tests Market
Influenza Diagnostic Tests Market Size
- Report ID: GMI4242
- Published Date: Dec 2023
- Report Format: PDF
Influenza Diagnostic Tests Market Size
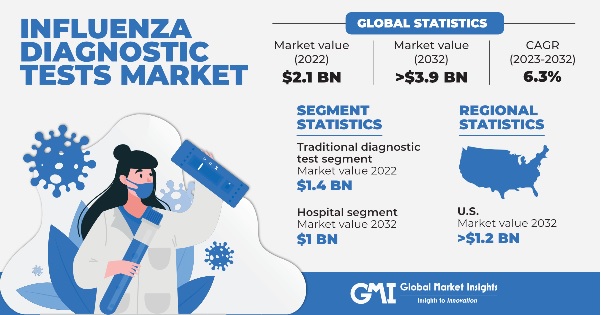
Influenza Diagnostic Tests Market size was valued at around USD 2.1 billion in 2022 and is estimated to witness 6.3% CAGR between 2023 and 2032. The growing prevalence of influenza, rising demand for rapid diagnostic tests, and increase in R&D activities are some of the key factors driving the market progression.
According to data from the World Health Organization, in 2023, there were around a billion cases of seasonal influenza annually, including 3–5 million cases of severe illness. Moreover, influenza causes 290,000 to 650,000 respiratory deaths annually around the globe. The rise in the incidence of influenza cases, especially during seasonal outbreaks, has led to an increased demand for accurate and rapid influenza diagnostic tests (RIDT) for timely identification and management of the infection. As a result, the rising influenza cases coupled with the high mortality rate are expected to drive the market progress.
Influenza diagnostic tests refer to a range of medical procedures and technologies designed to identify the presence of influenza viruses in individuals suspected of having an influenza infection. These tests play a crucial role in the timely and accurate diagnosis of influenza, allowing healthcare professionals to initiate appropriate interventions, treatment, and public health measures.
| Report Attribute | Details |
|---|---|
| Base Year: | 2022 |
| Influenza Diagnostic Tests Market Size in 2022: | USD 2.1 Billion |
| Forecast Period: | 2023 to 2032 |
| Forecast Period 2023 to 2032 CAGR: | 6.3% |
| 2032 Value Projection: | USD 3.9 Billion |
| Historical Data for: | 2018 to 2022 |
| No. of Pages: | 180 |
| Tables, Charts & Figures: | 291 |
| Segments covered: | Test Type, End-use, and Region |
| Growth Drivers: |
|
| Pitfalls & Challenges: |
|
The market faces the potential risk of hindered growth due to stringent regulatory guidelines. These guidelines are implemented to uphold the safety and efficacy of diagnostic solutions, often resulting in lengthy approval processes for new influenza diagnostic tests. Companies may experience delays in bringing their products to market due to the comprehensive evaluations required by regulatory agencies. This, in turn, can impede the timely availability of innovative diagnostic solutions, potentially hindering the business landscape.