Home > Healthcare > Medical Devices > Diagnostic Devices > HPV Testing and PAP Test Market
HPV Testing and PAP Test Market Size
- Report ID: GMI3174
- Published Date: May 2023
- Report Format: PDF
HPV Testing and PAP Test Market Size
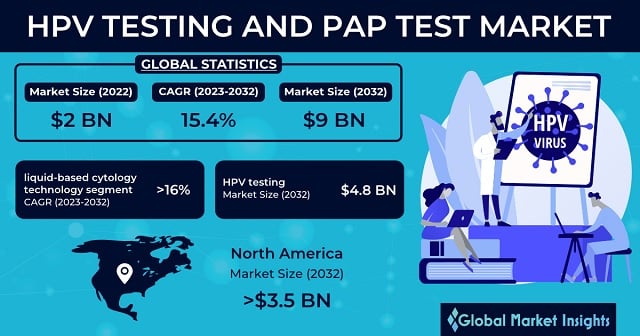
HPV Testing and PAP Test Market size was USD 2 billion in 2022 and is poised to observe over 15.4% CAGR from 2023-2032. Rising frequency of cervical cancer to drive global industry expansion.
The surging burden of cervical cancer worldwide will increase the demand for HPV testing and PAP tests. The growing popularity of screening test kits will further influence the industry expansion. To state an instance, Roche, in 2021, received the U.S. FDA approval for the Cobas HPV test deployed for detecting the risk of cervical cancer among women by identifying the presence of high-risk human papillomavirus (HPV) DNA in cervical samples.
| Report Attribute | Details |
|---|---|
| Base Year: | 2022 |
| HPV Testing and PAP Test Market Size in 2022: | USD 2 Billion |
| Forecast Period: | 2023 to 2032 |
| Forecast Period 2023 to 2032 CAGR: | 15.4% |
| 2032 Value Projection: | USD 9 Billion |
| Historical Data for: | 2018 to 2022 |
| No. of Pages: | 290 |
| Tables, Charts & Figures: | 253 |
| Segments covered: | Test Type, Technology, End-use, and Region |
| Growth Drivers: |
|
| Pitfalls & Challenges: |
|
The strong presence of stringent regulatory frameworks may act as a major restraint for the industry growth. The lack of uniformity within the regulatory frameworks is giving rise to uncertainty for several firms that develop and market HPV and PAP tests given the variation of requirements and standards offered by different regulatory bodies, further delaying the product developments and market approvals. The increasing complexity of these regulations will also limit the deployment of HPV and PAP testing.