Summary
Table of Content

Hemophilia Treatment Market
Get a free sample of this report
Form submitted successfully!
Error submitting form. Please try again.
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!

Request Sectional Data
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!
Form submitted successfully!
Error submitting form. Please try again.
Hemophilia Treatment Market Size
The global hemophilia treatment market size was valued at USD 15.2 billion in 2025. The market is expected to grow from USD 16 billion in 2026 to USD 27.2 billion in 2035, at a CAGR of 6.1% during the forecast period, according to the latest report published by Global Market Insights Inc.

To get key market trends
The market is experiencing increasing growth, primarily escalated due to the increasing prevalence of hemophilia A and B, along with the rising adoption of prophylactic therapies, also the introduction of advanced non-factor and gene therapies. For instance, Novo Nordisk mentions that 1 in 10,000 people globally suffer from hemophilia. Also, according to World Federation of Hemophilia, more than 233,577 hemophilia cases worldwide were found, stimulating the market demand.
Hemophilia treatment refers to therapeutics that are aimed at preventing or controlling bleeding episodes caused by deficiencies in clotting factors (Factor VIII or IX). These treatments include factor replacement therapies, non-factor replacement therapies, and gene therapies that offer potential long-term solutions. These interventions help maintain hemostasis, reduce complications, and improve quality of life for patients.
Key players stimulating the growth of the market include leading biopharmaceutical and healthcare companies such as Takeda Pharmaceuticals, CSL Behring, Pfizer, Sanofi, and F. Hoffmann-La Roche, which offer a wide range of recombinant factor concentrates, extended half-life products, and innovative gene therapy solutions. These companies play critical roles in product innovation, clinical trials, regulatory approvals, and expanding global access programs, thereby stimulating the adoption and expansion of hemophilia treatments worldwide.
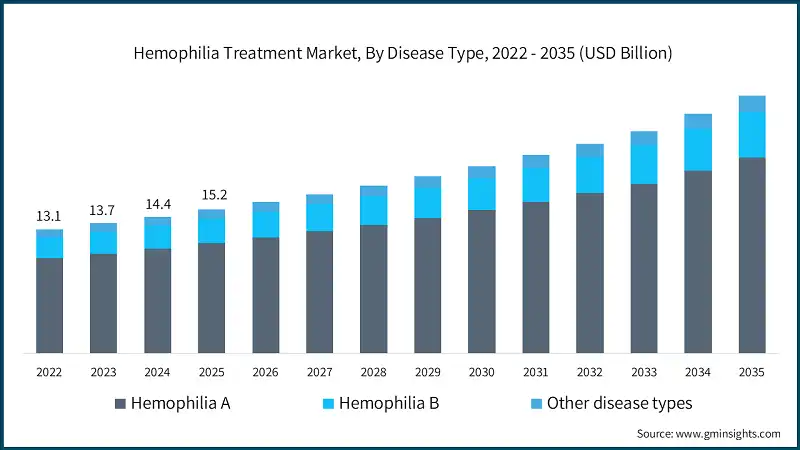
Between 2022 and 2024, the market witnessed considerable growth, driven by increased diagnosis rates, improved access to therapies in emerging markets, and the launch of novel non-factor therapies. For instance, the global market was valued at about USD 13.1 billion in 2022 and reached around USD 14.4 billion in 2024. This growth can be majorly attributed to the rising prevalence of hemophilia, the adoption of prophylactic regimens, and the availability of advanced recombinant and gene therapy products that reduce dependency on frequent infusions.
Hemophilia Treatment Market Report Attributes
| Key Takeaway | Details |
|---|---|
| Market Size & Growth | |
| Base Year | 2025 |
| Market Size in 2025 | USD 15.2 Billion |
| Market Size in 2026 | USD 16 Billion |
| Forecast Period 2026-2035 CAGR | 6.1% |
| Market Size in 2035 | USD 27.2 Billion |
| Key Market Trends | |
| Drivers | Impact |
| Increasing prevalence of hemophilia and bleeding disorders | Rising global incidence of hemophilia, A and B, coupled with improved survival rates, is driving sustained demand for lifelong treatment options. |
| Shift toward prophylactic and personalized therapies | Growing adoption of preventive regimens and tailored dosing strategies enhances patient outcomes and boosts market penetration. |
| Advancements in non-factor and gene therapies | Innovative treatments like emicizumab (NFRT) and AAV-based gene therapies are transforming care models, reducing infusion frequency, and improving quality of life. |
| Improved access and diagnosis in emerging markets | Expansion of screening programs and healthcare infrastructure in Asia-Pacific, Latin America, and Middle East is increasing treatment uptake. |
| Pitfalls & Challenges | Impact |
| High cost and affordability barriers | Gene therapies and extended half-life products remain extremely expensive, limiting access in low-income regions and straining reimbursement systems. |
| Complex regulatory and manufacturing requirements | Stringent compliance for biologics and gene therapies, coupled with supply chain vulnerabilities, can delay product launches and availability. |
| Opportunities: | Impact |
| Expansion of gene therapy and curative approaches | Increasing clinical success and regulatory approvals for one-time gene therapies will open new revenue streams and reduce long-term treatment burden. |
| Digital health integration and home-based care | Remote monitoring, telemedicine, and self-administration models will enhance patient convenience and drive adoption of subcutaneous and long-acting therapies. |
| Market Leaders (2025) | |
| Market Leaders |
15% market share |
| Top Players |
|
| Competitive Edge |
|
| Regional Insights | |
| Largest Market | North America |
| Fastest growing market | Asia Pacific |
| Emerging countries | China, India, Brazil, Mexico, South Africa |
| Future outlook |
|
What are the growth opportunities in this market?
Hemophilia Treatment Market Trends
- A key trend in the market is the ongoing shift from episodic, on demand infusions to proactive prophylaxis. Longer-acting recombinant factors and refined dosing protocols are enabling patients to maintain protective factor levels more consistently, reducing joint bleeds, preserving function, and aligning care with preventative medicine rather than crisis management.
- Moreover, non factor replacement therapies (NFRT) are redefining standards of care, particularly for Hemophilia A and patients with inhibitors. Subcutaneous agents that rebalance coagulation pathways offer convenient administration, extended dosing intervals, and robust bleed protection, easing the treatment burden and broadening suitability across age groups and clinical settings.
- Additionally, gene therapy is moving from promise to practice as a one time, potentially durable option, reshaping lifetime treatment economics and care models. As real world durability data mature and manufacturing scales, gene therapy is expected to lessen reliance on frequent infusions, compress long term costs, and shift follow up paradigms toward monitoring and optimization rather than chronic replacement.
- Further, personalized and data guided care is gaining traction, integrating pharmacokinetic profiling, digital adherence tools, and remote monitoring to tailor regimens. Individualized factor targets, bleed risk stratification, and home based reporting improve outcome predictability, minimizing under or over treatment, and strengthening shared decision making between clinicians and patients.
- Inhibitor management remains a core focus, with expanded use of immune tolerance induction and inhibitor agnostic therapies. Earlier detection, structured ITI protocols, and access to bypassing/NFRT options are reducing the clinical complexity of inhibitor cases and improving the likelihood of returning to effective prophylaxis.
- Also, home based and decentralized care models are accelerating, supported by subcutaneous administration, training programs, and telehealth. Self management, virtual check ins, and coordinated specialty pharmacy services are shortening clinic dependency, enhancing convenience, and sustaining high adherence in everyday life.
- Pipeline innovation remains vibrant across factor engineering, novel targets, and combination strategies. Efforts to further extend half life, refine subcutaneous options, and enhance gene delivery/immune control are expected to broaden choices, improve safety profiles, and support lifelong, stage appropriate therapy.
Hemophilia Treatment Market Analysis
Learn more about the key segments shaping this market
The global market was valued at USD 13.1 billion and USD 13.7 billion in 2022 and 2023, respectively. The market size reached USD 15.2 billion in 2025, growing from USD 14.4 billion in 2024.
Based on disease type, the global market is segmented into hemophilia A, hemophilia B, and other disease types. The hemophilia A segment has asserted its dominance in the market by securing a significant market share of 76.6% in 2025 and is anticipated to grow at a CAGR of 6% over the forecast years.
- The hemophilia A segment leads the market due to its high prevalence cases globally, according to NIH, hemophilia A it is the most common X-linked hereditary disorder of hemostasis, that represents nearly 80% of all hemophilia cases globally and is caused in 5,000 males. Factor VIII deficiency stimulates significant demand for factor replacement therapies, non-factor replacement therapies, and emerging gene therapies.
- Moreover, the increasing cases of mild and severe hemophilia A further stimulate the market demand, with Science Direct mentioning, with mild hemophilia A accounting for 6% to 40% of all the cases, further escalating the market demand.
- Additionally, strong clinical adoption of prophylactic regimens and innovative products such as extended half-life Factor VIII concentrates and subcutaneous non-factor therapies further reinforce its dominance. These treatments reduce bleeding episodes and improve joint health, making them the standard of care.
- The segment’s growth is further supported by rising diagnosis rates, improved access to advanced therapies in emerging markets, and continuous research and development investments in curative gene therapy approaches.
- Hemophilia B continues to hold a significant share of 16.8%, primarily stimulated by advancements in extended half-life Factor IX products and gene therapy breakthroughs. Hemophilia B accounts for a smaller patient population but benefits from high innovation activity, including one-time gene therapies that promise long-term factor expression and reduced treatment burden.
Based on the product, the global hemophilia treatment market is classified into recombinant factor concentrates, plasma-derived factor concentrates, extended half-life products, desmopressin, antifibrinolytic agents, gene therapy products and other products. The recombinant factor concentrates segment accounted for the highest market revenue of USD 7.1 billion in 2025 and is projected to grow at a CAGR of 6.2% during the analysis period.
- The recombinant factor concentrates are the preferred treatment option due to their superior safety profile, reduced risk of viral transmission, and consistent availability compared to plasma-derived alternatives. These products, including Factor VIII and Factor IX concentrates, are widely used in both on-demand and prophylactic regimens for hemophilia A and B.
- Moreover, the segment’s dominance is further escalated by continuous innovation in extended half-life recombinant factors, which reduce infusion frequency and improve patient adherence. Their proven efficacy in preventing bleeding episodes and preserving joint health makes them the cornerstone of hemophilia management globally.
- Also, the growing clinician preference for recombinant therapies, coupled with strong regulatory support and widespread reimbursement coverage, further stimulates adoption across developed and emerging markets.
- Likewise, the gene therapy products segment is anticipated to witness the fastest growth over the forecast period, driven by its potential to offer a one-time, long-lasting treatment solution.
Based on the patient, the global hemophilia treatment market is classified into pediatric and adult. The adult segment accounted for the highest market revenue of USD 8.8 billion in 2025 and is projected to grow at a CAGR of 6% during the analysis period.
- The adult segment is further segmented into 19 to 44 and 45+ age group.
- The high prevalence of hemophilia primarily stimulates the adults segment in the market. According to a study by Blood Transfusion IT, 75.3% prevalence of hemophilia was found in adults.
- Also, the increasing cases of severe hemophilia in adults is increasing with 2% to 10.8% prevalence, further stimulating the segment growth.
- Further, the high prevalence of hemophilia-related complications in older age groups and the need for long-term management of joint damage and inhibitor development also stimulate the market demand.
- In contrast, the pediatric segment is anticipated to witness the fastest growth over the forecast period, driven by early diagnosis and increasing adoption of preventive treatment strategies.
Based on treatment regime, the global hemophilia treatment market is classified into prophylaxis and on demand. The prophylaxis segment held a market share of 72.4% in 2025 and is projected to reach by USD 19.6 billion till 2035.
- Prophylaxis has become the standard of care for severe hemophilia patients, as it significantly reduces bleeding episodes, prevents joint damage, and improves long-term quality of life. According to a study by Springer, 70% used prophylaxis.
- The segment’s growth is further stimulated by the availability of extended half-life factor products and non-factor therapies that allow less frequent dosing and better adherence.
- On the other hand, the on-demand segment is anticipated to witness steady growth, supported by its continued use in mild or moderate hemophilia cases and resource-limited settings.
Based on the therapy, the global hemophilia treatment market is classified into factor replacement therapy and non-factor replacement therapy. The factor replacement therapy segment accounted for the highest market revenue of USD 13 billion in 2025 and is projected to grow at a CAGR of 5.9% during the analysis period.
- The Factor replacement therapy remains the cornerstone of hemophilia management, offering direct replacement of missing clotting factors. According to Hemophilia News Today, factor VIII (FVIII) replacement therapy for four years reduced annual bleeding rates by 91% for people with severe hemophilia A stimulating the market demand.
- Its dominance is escalated by decades of clinical use, proven efficacy in preventing and controlling bleeds, and widespread availability of recombinant and plasma-derived products.
- Continuous innovation in extended half-life factor concentrates and improved prophylactic protocols further strengthen the segment’s leadership globally.
- Likewise, the non-factor replacement therapy is anticipated to witness the fastest growth over the forecast period, stimulated by its convenience and efficacy in inhibitor patients.
Based on the route of administration, the global hemophilia treatment market is classified into injectable, nasal spray and oral. The injectable segment accounted for the highest market revenue of USD 12.8 billion in 2025 and is projected to grow at a CAGR of 5.9% during the analysis period.
- The injectable segment remains the standard route for hemophilia treatment, as most factor replacement therapies and gene therapy products require intravenous infusion for effective delivery.
- Its dominance is supported by decades of clinical practice, proven efficacy in controlling bleeds, and widespread availability of infusion-based products for both prophylaxis and on-demand regimens.
- Despite the emergence of alternative routes, injectable therapies continue to be the primary choice for severe hemophilia cases and complex treatment protocols.
- In contrast, the nasal spray segment is anticipated to witness the fastest growth over the forecast period, driven by its convenience and suitability for mild hemophilia cases.
Learn more about the key segments shaping this market
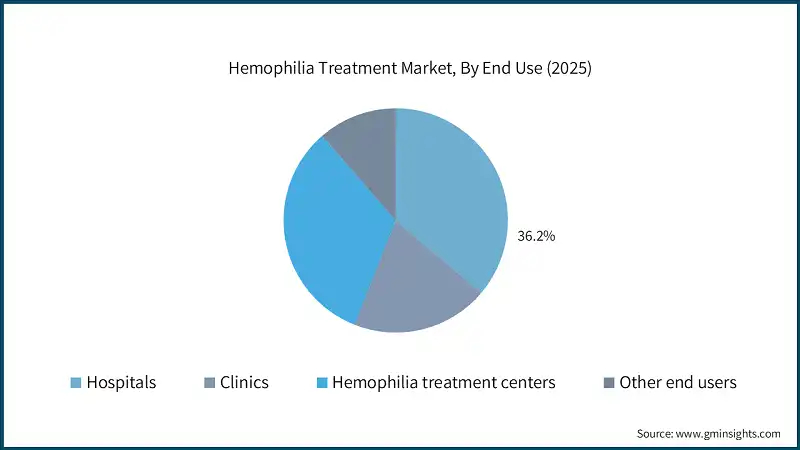
Based on end use, the global hemophilia treatment market is classified into hospitals, clinics, hemophilia treatment centers and other end users. The hospitals segment held a market share of 36.2% in 2025 and is projected to reach by USD 9.9 billion till 2035.
- Hospitals remain the primary treatment setting for hemophilia patients due to their advanced infrastructure, availability of specialized hematology units, and capacity to manage severe bleeding episodes and complex procedures.
- Its strong integration with infusion services, access to emergency care, and comprehensive diagnostic capabilities, making hospitals the first choice for both prophylactic and on-demand therapies.
- Moreover, high patient footfall established reimbursement systems, and availability of multidisciplinary care further keep hospitals leading position in the market.
- On the other hand, hemophilia treatment centers are anticipated to witness the fastest growth over the forecast period, stimulated by their specialized expertise and patient-centric care models.
Looking for region specific data?
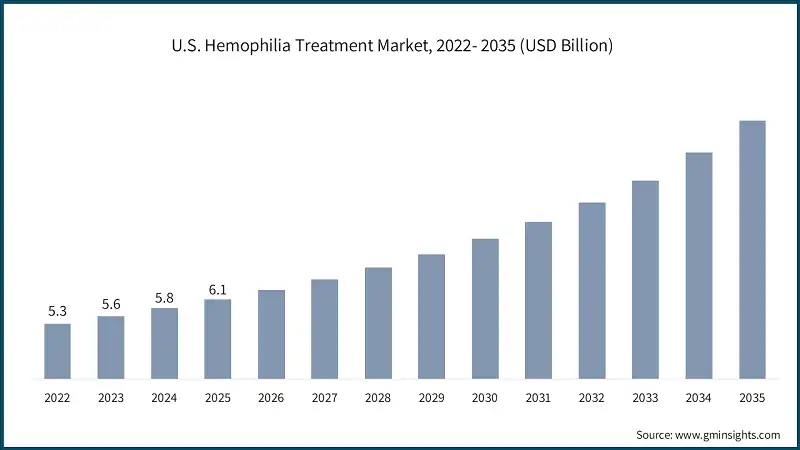
North America Hemophilia Treatment Market The North America market dominated the global market with a market share of 44.2% in 2025. The U.S. hemophilia treatment market was valued at USD 5.3 billion and USD 5.6 billion in 2022 and 2023, respectively. The market size reached USD 6.1 billion in 2025, growing from USD 5.8 billion in 2024. Europe market accounted for USD 3.6 billion in 2025 and is anticipated to show lucrative growth over the forecast period. Germany dominates the Europe hemophilia treatment market, showcasing strong growth potential. The Asia Pacific market is anticipated to grow at the highest CAGR of 6.6% during the analysis timeframe. China hemophilia treatment market is estimated to grow with a significant CAGR, in the Asia Pacific market. Brazil leads the Latin American market, exhibiting remarkable growth during the analysis period. Saudi Arabia market is expected to experience substantial growth in the Middle East and Africa market in 2025. The global market is moderately consolidated, with leading biopharmaceutical and specialty healthcare companies dominating the space, while innovation continues to thrive among emerging players. Top 5 companies such as Takeda Pharmaceutical Company Limited, CSL Behring, Novo Nordisk, Genentech (F. Hoffmann-La Roche), and Swedish Orphan Biovitrum AB (Sobi) collectively hold approximately 60% of the global market. These players are actively investing in research and development to expand their product portfolios, enhance treatment efficacy, and introduce next-generation therapies such as gene therapy and extended half-life factor products. The market also witnesses strategic collaborations, licensing agreements, and mergers and acquisitions aimed at accelerating the commercialization of innovative therapies and expanding geographic reach. Additionally, other prominent companies including Bayer Healthcare, Biogen, Biotest AG, Ferring B.V., Kedrion, Octapharma, Pfizer, Sanofi SA, BioMarin Pharmaceutical Inc., and others contribute significantly by focusing on specialized products and advanced treatment modalities. This dynamic environment fosters rapid technological advancements, competitive differentiation, and improved patient outcomes, driving the overall growth and diversification of the market. Prominent players operating in the hemophilia treatment industry are as mentioned below: Takeda Pharmaceutical is a leading player in the market, holding a significant share of 15% through its comprehensive portfolio of factor replacement therapies and innovative non-factor solutions. The company offers well-established products for Factor VIII replacement, alongside Feiba, a bypassing agent for inhibitor patients. Takeda’s competitive advantage lies in its strong focus on rare diseases, global distribution network, and continuous investment in next-generation therapies, including gene therapy platforms. CSL Behring maintains a dominant position in the market with a robust portfolio of plasma-derived and recombinant therapies. Key products which provide improved dosing convenience and clinical outcomes. The company leverages its expertise in plasma fractionation and biologics manufacturing, combined with strong global reach and strategic partnerships, to drive adoption across major markets. Novo Nordisk is a major contributor to the market, offering a diverse range of recombinant factor therapies and novel solutions for bleeding disorders. Its flagship products include NovoSeven RT, a recombinant Factor VIIa therapy widely used for inhibitor patients, and Esperoct, an extended half-life Factor VIII product. Novo Nordisk’s competitive edge stems from its strong clinical research capabilities, global presence, and emphasis on personalized treatment approaches.Europe Hemophilia Treatment Market
Asia Pacific Hemophilia Treatment Market
Latin American Hemophilia Treatment Market
Middle East and Africa Hemophilia Treatment Market
Hemophilia Treatment Market Share
Hemophilia Treatment Market Companies
Hemophilia Treatment Industry News
The hemophilia treatment market research report includes in-depth coverage of the industry with estimates and forecast in terms of revenue in USD Million from 2022 - 2035 for the following segments:
Market, By Disease Type
- Hemophilia A
- Severe
- Moderate
- Mild
- Hemophilia B
- Severe
- Moderate
- Mild
- Other disease types
Market, By Product
- Recombinant factor concentrates
- Factor VIII
- Factor IX
- Plasma-derived factor concentrates
- Factor VIII
- Factor IX
- Extended half-life products
- Factor VIII
- Factor IX
- Desmopressin
- Antifibrinolytic agents
- Gene therapy products
- Other products
Market, By Patient
- Pediatric
- 0 to 4
- 5 to 13
- 14 to18
- Adult
- 19-44
- 45+
Market, By Treatment Regime
- Prophylaxis
- On demand
Market, By Therapy
- Factor replacement therapy
- Non-factor replacement therapy
Market, By Route of Administration
- Injectable
- Nasal spray
- Oral
Market, By End Use
- Hospitals
- Clinics
- Hemophilia treatment centers
- Other end users
The above information is provided for the following regions and countries:
- North America
- U.S.
- Canada
- Europe
- Germany
- UK
- France
- Spain
- Italy
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East and Africa
- South Africa
- Saudi Arabia
- UAE
Frequently Asked Question(FAQ) :
Which product segment generated the highest revenue in 2025?
Recombinant factor concentrates generated the highest revenue, accounting for USD 7.1 billion in 2025, driven by superior safety profiles and widespread use in prophylactic regimens.
Which treatment regime leads the market?
The prophylaxis segment led the market with a 72.4% share in 2025, as it significantly reduces bleeding episodes, prevents joint damage, and improves long-term patient outcomes.
Which region leads the hemophilia treatment market?
North America led the global market with a 44.2% share in 2025, supported by advanced healthcare infrastructure, strong reimbursement systems, and early adoption of innovative therapies.
Who are the key players in the hemophilia treatment market?
Major players include Takeda Pharmaceutical, CSL Behring, Novo Nordisk, Genentech (F. Hoffmann-La Roche), Swedish Orphan Biovitrum (Sobi), Sanofi, and Pfizer.
Which disease type dominates the market?
Hemophilia A dominates the market, accounting for 76.6% share in 2025, due to its higher prevalence and strong clinical adoption of factor VIII replacement and non-factor therapies.
What is the market size of the hemophilia treatment market in 2025?
The global market was valued at USD 15.2 billion in 2025, driven by increasing prevalence of hemophilia A and B, rising diagnosis rates, and growing adoption of prophylactic therapies.
What will be the market value of the market in 2026?
The market is projected to reach USD 16 billion in 2026, reflecting steady growth driven by expanding prophylaxis adoption and improved access to advanced therapies.
What is the projected value of the hemophilia treatment market by 2035?
The market is expected to reach USD 27.2 billion by 2035, growing at a CAGR of 6.1% from 2026 to 2035, supported by advancements in non-factor therapies and gene therapy development.
Hemophilia Treatment Market Scope
Related Reports


