Home > Healthcare > Medical Devices > Therapeutic Devices > Enteral Feeding Devices Market
Enteral Feeding Devices Market Size
- Report ID: GMI745
- Published Date: Jun 2021
- Report Format: PDF
Enteral Feeding Devices Market Size
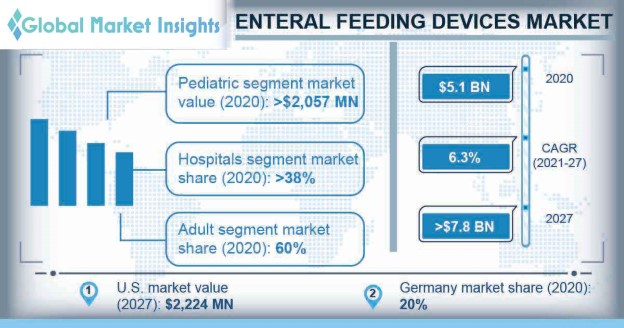
Enteral Feeding Devices Market size was valued at around USD 5.1 billion in 2020 and is estimated to grow at a CAGR of 6.3% between 2021 to 2027. Growing incidence of chronic diseases including cancer and neurological disorders such as stroke, multiple sclerosis, and dementia are stimulating the market growth. Expanding geriatric population base and rising number of people suffering from malnutrition are anticipated to increase the adoption of enteral feeding devices. In addition to this, rising prevalence of premature births in emerging economies is further expected to render significant impact, driving the market expansion.
Enteral feeding devices are used to intake food through the gastrointestinal (GI) tract with the help of a tube that passes directly to stomach or small intestine. These devices are advised for patients that have trouble ingesting vital amount of calories but can perform adequate gastrointestinal function to enable digestion and absorption. Enteral feeding devices finds extensive applications in intensive care unit (ICU), operation theatres (OT), critical care unit (CCU), and even in-home care settings.
| Report Attribute | Details |
|---|---|
| Base Year: | 2020 |
| Enteral Feeding Devices Market Size in 2020: | USD 5,120.7 million |
| Forecast Period: | 2021 to 2027 |
| Forecast Period 2021 to 2027 CAGR: | 6.3% |
| 2027 Value Projection: | USD 7,854.3 million |
| Historical Data for: | 2016 to 2020 |
| No. of Pages: | 300 |
| Tables, Charts & Figures: | 449 |
| Segments covered: | Product, Patient, Application, End-use and Region |
| Growth Drivers: |
|
| Pitfalls & Challenges: |
|
However, several complications associated with the use of enteral feeding devices on patient health may negatively impact the enteral feeding devices market share. Enteral feeding mechanism has been regularly associated with digestive complications such as diarrhoea, vomiting, abdominal distention, regurgitation and constipation. Mechanical complications related to these devices include tube obstruction, and aspiration pneumonia (bronchoaspiration) among others. Enteral feeding devices can also cause malnutrition due to feed intolerance and may result in a diminished response to disease and delayed recovery.
Furthermore, the U.S. FDA closely monitors the development, approval and post-approval adverse effects related to medical devices including enteral feeding devices. Owing to complications, misconnection injuries and dislodgment of tubes resulting in accidental disabilities and even deaths, these devices are classified under Class II medical devices. Majority of the Class II devices require a premarket notification (510k) process to get approval for commercial use of the product. The 510(k) approval represents a complicated process that demonstrates safety and efficacy of the device. The U.S.FDA monitors these devices to ensure optimum regulatory standards and to reduce the associated risks and injuries related to the device.
The COVID-19 pandemic has emerged as an unprecedented health concern that has rendered significant impact on the enteral feeding devices market. The coronavirus outbreak has affected millions of people across the globe causing significant mortalities. In order to curb the further transmission of SARS-CoV-2 virus, nationwide lockdowns were imposed, and emergencies had been declared in majority of the countries. The shutdown of major sectors of the economy distorted the supply chain and restricted the movement of raw materials, further limiting the industry growth. The COVID-19 affected the healthcare industry by financial adversities on the firms and by impacting production capacity. The production facilities were operating with lower employee strength further hampering the production and supply of enteral feeding equipment.
However, the use of enteral feeding tubes, especially in-home care settings is set to surge owing to pandemic. Heavy patient inflow and growing strain on healthcare system will push the use of enteral feeding tubes in home care settings in order to meet the nutritional needs of patients without visiting hospitals. New guidelines have been issued by the National Nurses Nutrition Group, for patients as well as medical professionals offering nutritional support with help of enteral tubes. Additionally, the European Society for Clinical Nutrition and Metabolism (ESPEN) published supportive information related to nutrition for severe COVID-19 patients. The published data suggests the use of enteral feeding equipment such as feeding tubes for meeting essential nutritional intake in severe COVID-19 patients. As a result, the pandemic will have a positive impact on the industry expansion.