Summary
Table of Content

Craniomaxillofacial Devices Market
Get a free sample of this report
Form submitted successfully!
Error submitting form. Please try again.
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!

Request Sectional Data
Thank you!
Your inquiry has been received. Our team will reach out to you with the required details via email. To ensure that you don't miss their response, kindly remember to check your spam folder as well!
Form submitted successfully!
Error submitting form. Please try again.
Craniomaxillofacial Devices Market Size
The global craniomaxillofacial devices market was valued at USD 2.8 billion in 2024. The market is expected to grow from USD 3.1 billion in 2025 to USD 5.1 billion in 2034, at a CAGR of 5.7% during the forecast period, according to the latest report published by Global Market Insights Inc. The high market growth is attributed to rising incidences of facial trauma and injuries, growing prevalence of congenital facial deformities, and expanding healthcare expenditure, among others.

To get key market trends
Craniomaxillofacial (CMF) devices are critical in modern surgical practice, enabling precise reconstruction and stabilization of facial and cranial structures following trauma, congenital deformities, or oncology-related resections. Leading manufacturers such as Stryker, Johnson & Johnson, KLS Martin Group, Zimmer Biomet, and B Braun offer a comprehensive portfolio that includes MF plate and screw fixation systems, cranial flap fixation systems, distraction devices, temporomandibular joint (TMJ) replacement systems, and bone graft substitutes. These solutions integrate advanced technologies such as patient-specific 3D printed implants, computer-assisted surgical planning, and bioresorbable materials, ensuring optimal anatomical fit, enhanced healing, and improved functional and aesthetic outcomes.
Craniomaxillofacial Devices Market Report Attributes
| Key Takeaway | Details |
|---|---|
| Market Size & Growth | |
| Base Year | 2024 |
| Market Size in 2024 | USD 2.8 Billion |
| Market Size in 2025 | USD 3.1 Billion |
| Forecast Period 2025 - 2034 CAGR | 5.7% |
| Market Size in 2034 | USD 5.1 Billion |
| Key Market Trends | |
| Drivers | Impact |
| Rising incidence of facial injuries and trauma | Increases demand for CMF implants and fixation systems. |
| Increasing adoption of cosmetic surgeries | Boosts utilization of CMF devices for aesthetic corrections. |
| Growing prevalence of congenital facial deformities | Drives reconstructive procedures requiring CMF solutions. |
| Expanding healthcare expenditure | Facilitates access to advanced CMF technologies globally. |
| Pitfalls & Challenges | Impact |
| High cost of craniomaxillofacial procedures | Limits adoption in price-sensitive and emerging markets. |
| Opportunities: | Impact |
| Growth in bioresorbable and biocompatible materials | Enhances product innovation and surgeon preference. |
| Expansion in emerging markets | Opens new revenue streams through increased surgical volumes. |
| Market Leaders (2024) | |
| Market Leaders |
~25.2% Market Share |
| Top Players |
Collective market share in 2024 is ~68.7% |
| Competitive Edge |
|
| Regional Insights | |
| Largest Market | North America |
| Fastest growing market | Asia Pacific |
| Emerging countries | China, India, South Korea, Brazil, and South Africa |
| Future outlook |
|
What are the growth opportunities in this market?
The market has increased from USD 1.9 billion in 2021 and reached USD 2.5 billion in 2023, with a historic growth rate of 15.3%. This expansion has been driven by rising adoption of advanced CMF implants, increasing trauma and reconstructive surgeries, and technological innovations such as patient-specific 3D-printed implants and computer-assisted surgical planning.
Growing facial injuries and trauma from road accidents, sports injuries, and violence are driving the market for craniomaxillofacial (CMF) devices due to increased demand for reconstructive surgeries, which is amplifying the need for reconstructive surgery. Additionally, an increase in congenital facial deformities in children will increase the need for CMF Devices in pediatric and reconstructive surgeries. Other key drivers include the increased difficulty of performing minimally invasive surgical procedures. These less intrusive surgeries are less traumatic, require shorter hospitalization periods, and offer quicker recovery, all of which support market growth.
Furthermore, adoption of new materials and imaging systems, which guarantee improved surgical results and enhanced patient protection, is sharply on the rise among healthcare professionals. Increasing healthcare spending in developing countries, as well as progressive policies regarding reimbursement, are expanding access to these devices. As patients with complex challenges seeking facial reconstruction become more obvious, these trends will accelerate the growth of the CMF devices market worldwide.
Craniomaxillofacial (CMF) devices are medical implants and surgical devices used to repair, reconstruct, or stabilize the bones of the skull, face, and jaw.
Craniomaxillofacial Devices Market Trends
The increasing adoption of cosmetic and aesthetic surgeries is one of the major factors driving the demand for the market.
- Over the past decade, the perception of cosmetic procedures has evolved from being luxury-oriented to a more mainstream healthcare practice focused on improving appearance, confidence, and facial harmony. This rising trend has significantly expanded the use of CMF devices in reconstructive and elective facial procedures.
- The growing social awareness, media influence, and demand for facial symmetry have significantly increased the adoption of cosmetic facial procedures. For instance, according to the American Society of Plastic Surgeons (ASPS), there was a 5% rise in plastic surgeries and a 7% rise in minimally invasive cosmetic procedures compared to the previous year.
- Additionally, the International Society of Aesthetic Plastic Surgery (ISAPS) reported a 3.4% increase in total surgical and non-surgical cosmetic procedures in 2023, reaching 34.9 million. Craniomaxillofacial (CMF) devices, including plates, screws, and fixation systems, are essential in facial contouring, jawline reconstruction, and chin augmentation procedures, as they enhance aesthetics while maintaining structural integrity.
- Furthermore, the American Academy of Facial Plastic and Reconstructive Surgery (AAFPRS) noted a 40% increase in chin augmentation procedures in 2021, with the upward trend continuing through 2023.
- Moreover, the development of advanced imaging, 3D printing, and patient-specific implant (PSI) technologies has revolutionized facial cosmetic surgeries. Surgeons can now plan procedures with greater precision, ensuring personalized outcomes and shorter recovery times. These technological advancements have made aesthetic surgeries safer and more appealing, thereby driving the adoption of CMF devices.
Craniomaxillofacial Devices Market Analysis
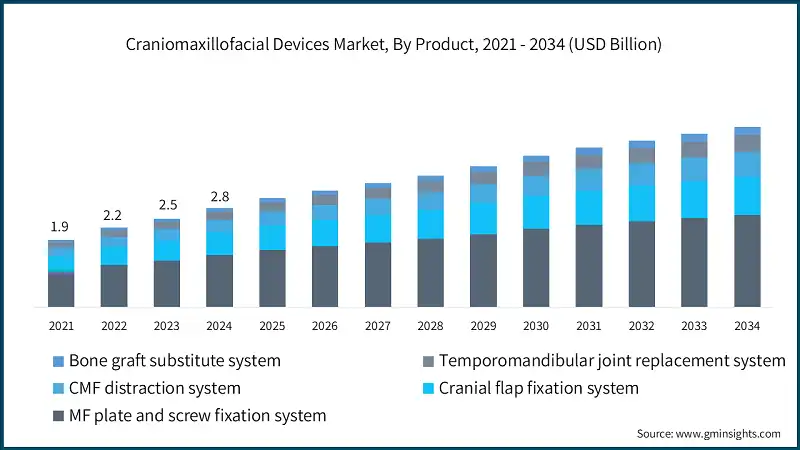
Learn more about the key segments shaping this market
Based on product, the craniomaxillofacial devices market is segmented into MF plate and screw fixation system, cranial flap fixation system, CMF distraction system, temporomandibular joint replacement system, and bone graft substitute system. The MF plate and screw fixation system segment accounted for 52.4% of the market in 2024 due to its widespread use in trauma and reconstructive surgeries, versatility across multiple craniomaxillofacial applications, and strong adoption of advanced plating technologies such as 3D-printed and patient-specific solutions. The segment is expected to exceed USD 2.6 billion by 2034, growing at a CAGR of 5.5% during the forecast period.
On the other hand, the cranial flap fixation system segment held a market share of 22.6% in 2024, and its growth can be attributed to the rising number of neurosurgical procedures, increasing adoption of advanced fixation technologies for craniotomies, and growing preference for titanium and bioresorbable systems that ensure secure closure and reduce postoperative complications.
- The MF plate and screw fixation system remains one of the most popular systems in use today because of its dependability in craniomaxillofacial surgeries. These systems provide reliable fixation for complex fractures involving the mandible, midface, and other pivotal areas of the skull. Owing to the straightforward nature of their application and their capability to enable accurate alignment of bone fragments, MF Plate and Screw Fixation Systems are preferred by most surgeons. They maximize effective stabilization, enhance healing, and reduce recovery times.
- The CMF distraction system segment was valued at USD 364.8 million in 2024 and is expected to reach USD 696.3 million by 2034. Craniomaxillofacial (CMF) distraction devices are essential for treating conditions such as cleft lip/palate, craniosynostosis, and micrognathia. According to the Centers for Disease Control and Prevention (CDC), approximately 1 in every 1,050 babies is born with a cleft lip with or without a cleft palate, and about 1 in every 1,600 babies is born with a cleft palate alone (as of 2024). Additionally, the CDC estimates that congenital anomalies occur in about 1 in every 33 infants born in the U.S. each year, with craniofacial deformities among the most common.
- The temporomandibular joint replacement system segment held a revenue of USD 219 million in 2024, with projections indicating a steady expansion at 7.7% CAGR from 2025 to 2034. Rising cases of TMJ disorders, osteoarthritis, ankylosis, and trauma-related injuries are increasing the demand for replacement procedures. For example, according to the National Institute of Dental and Craniofacial Research, approximately 10 million Americans were affected by TMJ disorders in 2023.
- The bone graft substitute system segment held a revenue of USD 113 million in 2024, with projections indicating a steady expansion at 6.9% CAGR from 2025 to 2034. Traditional autografts require harvesting bone from the patient, leading to donor site morbidity and extended operative time. Bone graft substitutes, including allografts, xenografts, and synthetic materials, eliminate these drawbacks. This growing preference for less invasive, off-the-shelf solutions drives the adoption of bone graft substitutes in CMF procedures.
Based on implant type, the craniomaxillofacial devices market is segmented into standard implants and customized/patient-specific implants. The standard implants segment dominated the market in 2024 and is growing with a CAGR of 5.2% during the forecast period.
- Standard implants, including preformed plates, screws, and meshes, are widely used in routine facial fracture repair, orthognathic surgery, and minor reconstructive procedures. Their ready-to-use design allows quick application during surgery, making them essential in general CMF practice. High procedural volumes worldwide support steady demand.
- Additionally, these implants are versatile and compatible with a variety of surgical approaches, including open reduction, internal fixation, and minimally invasive procedures. Their adaptability across different facial regions and fracture types increases their utility and adoption.
- The customized/patient-specific implants segment accounted for USD 895.5 million in 2024 and is expected to grow at a 6.8% CAGR from 2025 to 2034. The customized/patient-specific implants (PSIs) segment is witnessing strong growth, driven by rising demand for anatomical accuracy and aesthetic outcomes, the increasing prevalence of complex craniofacial deformities, integration with advanced surgical planning and navigation, and innovations in materials and manufacturing.
Based on location, the craniomaxillofacial devices market is segmented into internal fixators and external fixators. The internal fixators segment dominated the market in 2024 and is growing with a CAGR of 5.4% during the forecast period.
- The increasing incidence of complex midface and mandibular fractures is driven by road accidents, sports injuries, and falls. For instance, according to the National Highway Traffic Safety Administration, 42,915 people died in motor vehicle traffic crashes last year, a 10.5% increase from 38,824 fatalities in 2020.
- Internal fixators allow immediate stabilization of facial bones, promoting early jaw mobility and a quicker return to normal function. This reduces the risk of malocclusion, soft tissue complications, and muscle atrophy, making them increasingly preferred by surgeons and patients for better postoperative outcomes.
- The external fixators segment is expected to grow at the highest pace during the period from 2025 to 2034 at a CAGR of 7%. External fixators allow gradual reduction and alignment of complex fractures, including panfacial or midface injuries. They can be adjusted postoperatively to correct malocclusion or asymmetry, providing flexibility that internal fixation cannot. This adaptability makes them essential for managing.
- These devices can be applied without extensive surgical exposure, preserving soft tissue and reducing the risk of infection in contaminated wounds. This feature is particularly valuable in open fractures or post-tumor resections, making them a key driver of adoption.
Based on metals, the craniomaxillofacial devices market is segmented into metals, bioabsorbable materials, ceramics, and polymers. The metals segment continues to dominate, accounting for a majority market share valued at USD 1.6 billion in 2024.
- Metals such as titanium and stainless steel offer excellent strength, stiffness, and load-bearing capacity, which is critical for stabilizing craniofacial bones. Their durability ensures long-term structural integrity, especially in high-stress regions like the mandible and midface. The mechanical reliability of metals drives their continued preference over resorbable materials in certain procedures.
- The bioabsorbable materials segment was valued at USD 696.3 million in 2024. Bioabsorbable materials are gaining traction in the craniomaxillofacial (CMF) devices market due to their ability to provide temporary mechanical support while gradually degrading within the body, eliminating the need for secondary surgeries to remove implants.
- The ceramics segment held a revenue of USD 301.8 million in 2024, with projections indicating a steady expansion at 5.9% CAGR from 2025 to 2034. Ceramics are emerging as a specialized segment in the market due to their exceptional biocompatibility, chemical stability, and resistance to wear and corrosion.
- Materials such as hydroxyapatite and alumina are commonly used in craniofacial reconstruction because they closely mimic the mineral composition of natural bone, promoting osteointegration and reducing the risk of immune rejection.
- The polymers segment held a revenue of USD 167.4 million in 2024, with projections indicating a steady expansion at 7.6% CAGR from 2025 to 2034. Polymers are increasingly utilized in the craniomaxillofacial (CMF) devices market owing to their lightweight nature, flexibility, and versatility in design.
- Materials such as polyethylene, polyetheretherketone (PEEK), and polymethylmethacrylate (PMMA) are favored for patient-specific implants and reconstructive procedures, offering ease of contouring and customization.
Based on application, the craniomaxillofacial devices market is segmented into trauma reconstruction surgery, orthognathic surgery, and plastic surgery. The trauma reconstruction surgery segment continues to dominate, securing a majority market share valued at USD 1.4 billion in 2024.
- The global rise in road traffic accidents, sports injuries, and occupational hazards has led to an increase in complex facial fractures requiring reconstruction. Devices such as Johnson & Johnson CMF plates and screws provide rigid fixation for mandibular, midface, and orbital fractures, and the growing trauma burden drives their adoption.
- The orthognathic surgery segment held a revenue of USD 1 billion in 2024, with projections indicating a steady expansion at 6.4% CAGR from 2025 to 2034.
- The rising prevalence of jaw misalignment, facial asymmetry, and malocclusion is increasing the demand for orthognathic surgeries. For instance, the American Association of Oral and Maxillofacial Surgeons reported that 22% of patients seeking orthodontic treatment in 2023 required surgical intervention.
- The plastic surgery segment held a revenue of USD 329.3 million in 2024, with projections indicating a steady expansion at 4.3% CAGR from 2025 to 2034. The plastic surgery segment in the craniomaxillofacial (CMF) devices market is growing steadily, driven by the increasing demand for facial reconstruction, aesthetic enhancement, and correction of congenital deformities. CMF devices play a crucial role in procedures such as facial contouring, chin and cheek augmentation, and reconstruction following trauma or tumor resection.
Based on resorbability, the craniomaxillofacial devices market is segmented into non-resorbable fixators and resorbable fixators. The non-resorbable fixators segment dominated the market in 2024 and is growing with a CAGR of 5.4% during the forecast period.
- Non-resorbable fixators hold a significant share in the market owing to their superior mechanical strength, durability, and reliability in stabilizing complex fractures. Made primarily from titanium and its alloys, these fixators provide long-term structural support and are ideal for load-bearing applications such as mandibular and midface reconstruction.
- Their excellent biocompatibility and corrosion resistance minimize adverse tissue reactions and ensure sustained performance over time.
- The resorbable fixators segment is expected to grow at a substantial pace during the period from 2025 to 2034 at a CAGR of 6.8%. Resorbable fixators made from polymers such as polylactic acid (PLA), polyglycolic acid (PGA), and polycaprolactone (PCL) are gaining traction due to their superior biocompatibility and reduced risk of long-term complications.
- Unlike metallic plates, they naturally degrade within the body, eliminating the need for secondary implant removal. This biocompatible advantage strongly drives adoption.
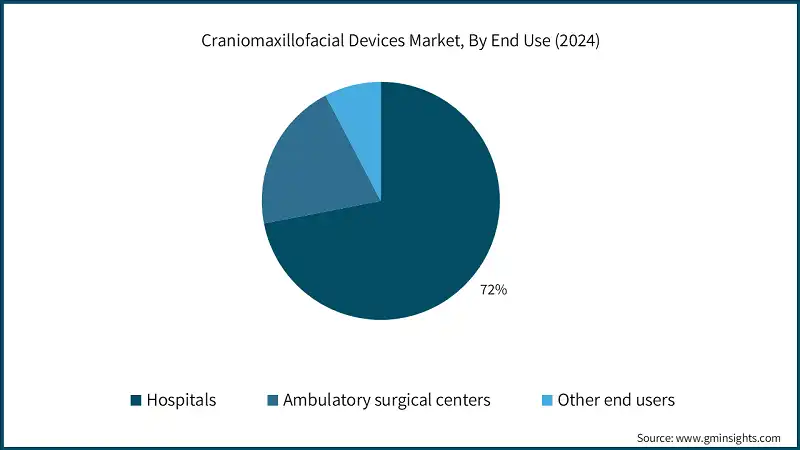
Learn more about the key segments shaping this market
Based on end use, the craniomaxillofacial devices market is segmented into hospitals, ambulatory surgical centers, and other end users. The hospitals segment accounted for USD 2 billion in 2024 and is expected to grow at a 5.6% CAGR from 2025 to 2034.
- Hospitals handle the majority of complex CMF procedures, including trauma reconstruction, orthognathic surgery, and tumor resection. These surgeries require advanced equipment, multidisciplinary teams, and postoperative care facilities available only in hospital settings. This concentration of complex cases drives higher utilization of CMF devices in hospitals compared to other settings.
- The ambulatory surgery centers segment accounted for USD 566.5 million in 2024 and is expected to grow at a 6.8% CAGR from 2025 to 2034. Ambulatory surgery centers (ASCs) are emerging as a key channel in the craniomaxillofacial (CMF) devices market, driven by the increasing preference for outpatient procedures that offer cost efficiency, shorter recovery times, and patient convenience.
- ASCs are increasingly performing CMF surgeries such as mandibular reconstruction, orbital floor repair, and cranial defect correction, supported by advanced fixation systems, bioabsorbable implants, and patient-specific devices.
- The other end users segment is expected to grow at a substantial pace during the period from 2025 to 2034 at a CAGR of 4.4%. The other end users include specialized medical facilities, research institutions, and rehabilitation centers, representing a growing avenue for device utilization.
- The rising awareness of multidisciplinary approaches in craniofacial care and the demand for tailored, patient-specific treatments are expanding the role of these end users, supporting overall market growth beyond traditional hospital and ASC settings.
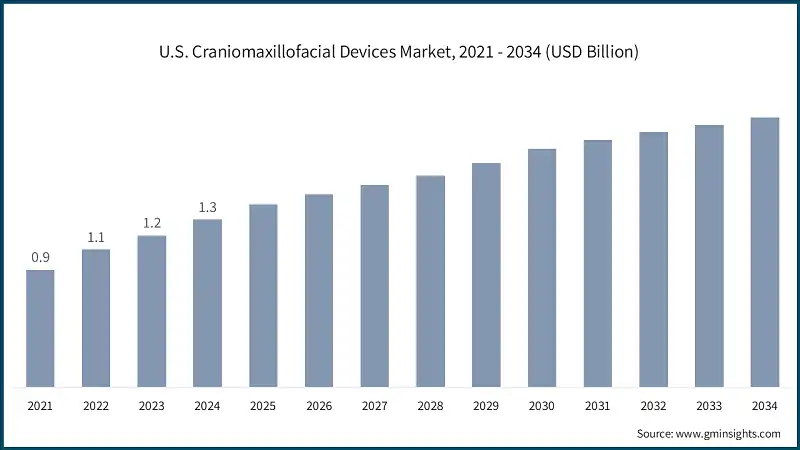
Looking for region specific data?
North America Craniomaxillofacial Devices Market
North America dominated the global market with the highest market share of 49.7% in 2024.
- The U.S. market was valued at USD 907.2 million and USD 1.1 billion in 2021 and 2022, respectively. The market size reached USD 1.3 billion in 2024, growing from USD 1.2 billion in 2023, and is anticipated to grow at a CAGR of 4.4% between 2025 and 2034.
- The U.S. has a considerable portion of the craniomaxillofacial devices market due to a significant number of automotive and sports-associated accidents mandatorily requiring reconstructive surgery.
- The improvement of healthcare access through government programs and further innovation in technologies such as medical devices increases the use of CMF devices in hospitals and healthcare centers.
- North America is home to several global CMF device companies, including Stryker, Zimmer Biomet, and Johnson & Johnson. Their extensive distribution networks, diverse product portfolios, and ongoing R&D activities enhance availability and adoption, supporting market growth.
Europe Craniomaxillofacial Devices Market
Europe market accounted for USD 785.7 million in 2024 and is anticipated to show lucrative growth over the forecast period.
- Europe maintains an extensive network of tertiary care hospitals and craniofacial centers that use advanced surgical technologies, including 3D imaging, virtual planning, and intraoperative navigation.
- According to the European Commission's Healthcare Resource Statistics 2023, the number of specialized surgical centers performing craniomaxillofacial procedures increased by 15% between 2021 and 2023.
- This medical infrastructure enables complex craniomaxillofacial (CMF) procedures, including trauma reconstruction, orthognathic surgery, and temporomandibular joint (TMJ) replacement, increasing the demand for CMF devices.
- Many European countries offer reimbursement and insurance coverage for craniofacial reconstructive, trauma, and orthognathic procedures. This financial support enables hospitals and patients to access high-cost CMF devices such as resorbable fixators and custom implants, facilitating broader market adoption.
Asia Pacific Craniomaxillofacial Devices Market
The Asia Pacific market is anticipated to grow at a CAGR of 8.5% during the analysis timeframe.
- The Asia-Pacific region’s large population experiences a high incidence of craniofacial trauma. In India, the Ministry of Road Transport and Highways reported 461,312 road accidents in 2022, resulting in 168,491 deaths, of which around 40% involved head injuries.
- This trauma burden has increased the demand for craniomaxillofacial (CMF) devices, such as plates, screws, and fixation systems, in hospitals and trauma centers for fracture treatment.
- Patients and surgeons in countries such as China, India, and Japan are becoming increasingly aware of the functional and aesthetic benefits of CMF reconstruction, orthognathic, and TMJ surgeries. This rising awareness fuels demand for standard, patient-specific implants and advanced fixation systems.
- Several Asia-Pacific countries are expanding healthcare spending, improving hospital infrastructure, and subsidizing reconstructive surgeries. These initiatives make high-cost CMF devices more accessible in public hospitals, increasing device penetration in both urban and semi-urban regions.
Latin America Craniomaxillofacial Devices Market
The Latin America market is experiencing robust growth over the analysis timeframe.
- The Latin America craniomaxillofacial (CMF) devices market is witnessing steady growth, driven by increasing incidences of facial trauma, congenital deformities, and reconstructive surgical procedures across the region.
- Countries such as Brazil, Mexico, and Argentina are investing in advanced healthcare infrastructure, expanding access to specialized craniofacial surgery and aesthetic procedures. Rising awareness among patients regarding facial reconstruction and cosmetic enhancement, coupled with growing medical tourism, is further fueling demand for CMF devices.
- Adoption of technologically advanced solutions, including bioabsorbable implants, 3D-printed patient-specific devices, and minimally invasive fixation systems, is gradually increasing in major urban centers.
- Additionally, supportive government initiatives and healthcare reforms aimed at improving surgical care accessibility are encouraging the use of advanced CMF implants in hospitals and specialized clinics.
Middle East & Africa Craniomaxillofacial Devices Market
The Middle East & Africa market is experiencing notable growth over the analysis timeframe.
- The MEA region is expanding due to rising facial trauma cases, road accidents, and growing demand for reconstructive and aesthetic surgeries. Investments in healthcare infrastructure, particularly in the UAE, Saudi Arabia, and South Africa, are enhancing access to advanced CMF procedures.
- Increasing adoption of patient-specific implants, bioabsorbable materials, and minimally invasive fixation systems supports improved surgical outcomes and faster recovery.
- Additionally, medical tourism, rising awareness of facial reconstruction options, and government initiatives to modernize healthcare facilities are driving market growth. The region’s focus on technologically advanced and cost-effective solutions is expected to sustain CMF device adoption.
Craniomaxillofacial Devices Market Share
Leading industry players such as Stryker, Johnson & Johnson, KLS Martin Group, Zimmer Biomet, and B Braun collectively hold a market share of around 68.7% in the fragmented market. These firms maintain their dominance through innovation in 3D printing, bioresorbable materials, and digital surgical planning that enhance surgical precision and patient outcomes. Established companies leverage global distribution networks, surgeon training programs, and regulatory expertise to maintain leadership.
Meanwhile, emerging players focus on niche applications such as customized cranial implants and resorbable fixators, creating opportunities for differentiation. Strategic collaborations with hospitals, research institutions, and digital technology providers are further shaping market growth. Overall, the CMF devices market is shifting toward personalized, minimally invasive, and digitally integrated solutions, intensifying competition and driving continuous improvement in device performance and surgical efficiency.
Craniomaxillofacial Devices Market Companies
A few of the prominent players operating in the craniomaxillofacial devices industry include:
- Acumed
- Anatomics
- B BRAUN
- Beijing Naton Medical Technology
- BIOPLATE
- Cavendish Implants
- CranioTech
- JEIL MEDICAL
- Johnson & Johnson
- Kelyniam
- KLS Martin Group
- Matrix Surgical USA
- Medartis
- MEDPRIN
- Medtronic
- Stryker
- Xilloc Medical
- Zimmer Biomet
- Stryker
Stryker is a global leader in the craniomaxillofacial devices market, offering a broad range of fixation systems, including titanium plates, mesh, and resorbable implants. The company’s CMF Facial iD, Leibinger, and 3D-printed cranial implant lines are widely adopted for trauma, orthognathic, and reconstructive procedures.
Stryker’s focus on digital surgical planning and customization enhances surgical precision and patient outcomes. The company also invests heavily in surgeon education, ensuring safe and efficient implant use. With its strong global footprint and continuous innovation, Stryker remains a top choice among healthcare professionals for CMF solutions.
DePuy Synthes, part of Johnson & Johnson MedTech, is a major force in the craniomaxillofacial devices space. The company’s MatrixMANDIBLE, MatrixMIDFACE, and MatrixNEURO systems are used worldwide for facial fracture fixation and reconstructive surgery. These devices are valued for their ergonomic design, biomechanical stability, and compatibility with advanced imaging systems. DePuy Synthes focuses on procedural efficiency and reducing operative time through system integration and standardized instrumentation. With its established brand trust, clinical validation, and comprehensive product support, the company continues to lead innovation in CMF surgery.
KLS Martin Group is renowned for its specialized expertise in craniomaxillofacial surgery, offering an extensive range of plates, screws, distraction systems, and resorbable materials. Its IPS Implants (Individual Patient Solutions) leverage cutting-edge 3D printing technology to create tailored implants for complex cranial and facial reconstructions. The company’s close collaboration with clinicians ensures that designs are both anatomically accurate and functionally optimized. KLS Martin emphasizes superior craftsmanship, biocompatibility, and innovation, making it a preferred partner for advanced reconstructive and aesthetic craniofacial procedures across global markets.
Craniomaxillofacial Devices Industry News:
- In May 2022, Medartis AG completed the acquisition of Nextremity Solutions Inc., strengthening its extremities and market. The deal brings advanced product pipelines, strong surgeon partnerships, and modern manufacturing facilities.
- In August 2022, Stryker opened its high-tech facility at Anngrove, enhancing its additive manufacturing (3D printing) capabilities for healthcare innovation. The facility supports the production of specialized medical devices, including CMF implants and patient-specific solutions, improving access to advanced implants and accelerating innovation in reconstructive surgeries.
The craniomaxillofacial devices market research report includes an in-depth coverage of the industry with estimates and forecasts in terms of revenue in USD Million from 2021 - 2034 for the following segments:
Market, By Product
- MF plate and screw fixation system
- Cranial flap fixation system
- CMF distraction system
- Temporomandibular joint replacement system
- Bone graft substitute system
Market, By Implant Type
- Standard implants
- Customized/patient-specific implants
Market, By Location
- Internal fixators
- External fixators
Market, By Material
- Metals
- Bioabsorbable materials
- Ceramics
- Polymers
Market, By Application
- Trauma reconstruction surgery
- Cranial surgery
- Mid-face surgery
- Mandibular surgery
- Orbital floor reconstruction surgery
- Orthognathic surgery
- Plastic surgery
Market, By Resorbability
- Non- resorbable fixators
- Resorbable fixators
Market, By End Use
- Hospitals
- Ambulatory surgery centers
- Other end use
The above information is provided for the following regions and countries:
- North America
- U.S.
- Canada
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Netherlands
- Switzerland
- Belgium
- Sweden
- Poland
- Austria
- Denmark
- Ireland
- Portugal
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Thailand
- Latin America
- Brazil
- Mexico
- Argentina
- Colombia
- Chile
- Middle East and Africa
- South Africa
- Saudi Arabia
- UAE
Frequently Asked Question(FAQ) :
Which region leads the craniomaxillofacial devices market?
North America led the market with a 49.7% share in 2024, driven by advanced healthcare infrastructure and high adoption of cosmetic and reconstructive surgeries.
Who are the key players in the craniomaxillofacial devices market?
Key players include Acumed, Anatomics, B BRAUN, Beijing Naton Medical Technology, BIOPLATE, Cavendish Implants, CranioTech, JEIL MEDICAL, Johnson & Johnson, and Kelyniam.
How much revenue did the MF plate and screw fixation system segment generate?
The MF plate and screw fixation system segment accounting for 52.4% of the market share, driven by its versatility and adoption of advanced technologies like 3D printing.
What are the upcoming trends in the craniomaxillofacial devices industry?
Key trends include the increasing adoption of cosmetic and aesthetic surgeries, advancements in patient-specific implants, and the integration of 3D printing technologies in device manufacturing.
What is the market size of the craniomaxillofacial devices in 2024?
The market size was USD 2.8 billion in 2024, with a CAGR of 5.7% expected through 2034, driven by rising incidences of facial trauma, congenital facial deformities, and increasing healthcare expenditure.
What was the valuation of the standard implants segment?
The standard implants segment dominated the market in 2024, growing at a CAGR of 5.2% during the forecast period.
What is the projected size of the craniomaxillofacial devices market in 2025?
The market is expected to reach USD 3.1 billion in 2025.
What is the projected value of the craniomaxillofacial devices market by 2034?
The market is expected to reach USD 5.1 billion by 2034, supported by advancements in plating technologies, growing adoption of cosmetic surgeries, and demand for patient-specific implants.
Craniomaxillofacial Devices Market Scope
Related Reports


