Home > Healthcare > Medical Devices > Diagnostic Devices > Blood Testing Market
Blood Testing Market Analysis
- Report ID: GMI2683
- Published Date: Jul 2023
- Report Format: PDF
Blood Testing Market Analysis
Blood testing market share from the complete blood count test segment is projected to reach USD 20.5 billion by 2032 attributed to the increasing prevalence of non-communicable diseases (NCDs). According to the WHO, cardiovascular diseases account for majority of NCD mortality (17.9 million), followed by cancer (9.3 million), respiratory ailments (4.1 million), and diabetes (1.5 million). Blood tests are crucial in diagnosing and monitoring a wide range of diseases and conditions such as anemia, infections, and certain cancers. The rising awareness about preventive healthcare and higher advancements in laboratory technologies will also fuel the segment trends.

On the basis of product, the blood testing market size from the reagents & kits segment is anticipated to witness 6.5% CAGR from 2023-2032 owing to the rising number of blood transfusions. Moreover, the growing prevalence of infectious diseases, chronic conditions, and genetic disorders along with the increasing number of organ transplantations and blood transfusions will stir the need for specialized reagents and kits to cater to specific tests.
Based on end-use, the hospitals segment held 47.3% share of the blood testing market in 2022 and is projected to amass substantial growth from 2023-2032 due to the increasing number of hospital admissions and longer duration of patient stays. With rising technological advancements and higher availability of a wide range of blood tests, hospitals offer access to more accurate and efficient diagnostic tools. This has led to increased adoption of blood testing services in hospitals for facilitating early detection, personalized treatment plans and improved patient outcomes, boosting the segment development.
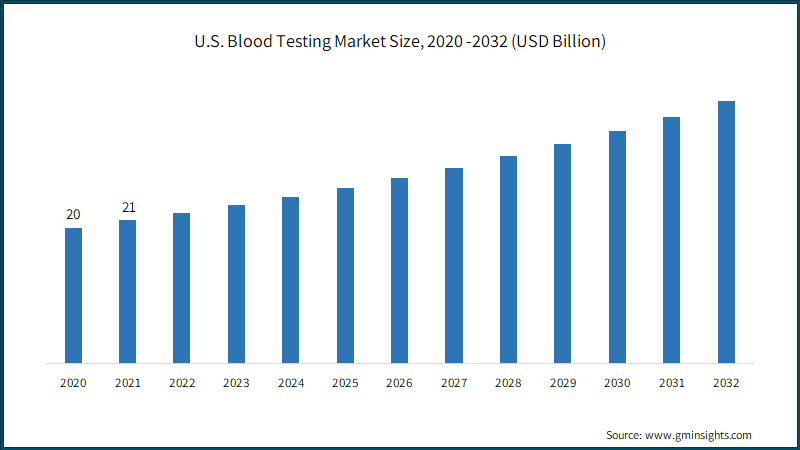
North America blood testing market size is estimated to reach USD 43.4 billion by 2032. The regional market growth is attributed to the strong presence of favorable reimbursement policies, robust healthcare infrastructure, supportive government initiatives, and Clinical Laboratory Improvement Amendments (CLIA) exemptions for testing devices. The emphasis on preventive healthcare and early disease detection along with the introduction of diagnostic devices will also enhance the market penetration in the region.

